Label: NUTRICEL ADDITIVE SOLUTION - CP2D- cp2d/as-3 anticoagulant and additive system solution
NUTRICEL ADDITIVE SOLUTION - AS-3- cp2d/as-3 anticoagulant and additive system solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 53157-100-94, 53157-100-95, 53157-100-96, 53157-101-97, view more53157-101-98, 53157-101-99 - Packager: Haemonetics Manufacturing Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 13, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
CP2D/AS-3 Blood Collection and Sampling System – Nutricel Additive System
Instruction for Use for Systems Containing a Y Sampling Site (YSS) or Sample Diversion Pouch (with or without a pre-attached SampLok® Vacuum Tube Holder).
Refer to unit foil package label for specific product description being used.
- INDICATIONS AND USAGE
- WARNINGS
-
GENERAL PRECAUTIONS
Use aseptic technique. Use only if solutions are clear. If preparing a platelet concentrate, the platelet-rich plasma should be separated from the red blood cells within 8 hours after blood collection. If preparing fresh frozen plasma, separate from the red blood cells and place in the freezer at -18 °C or colder within 8 hours after collection.
* During processing, always observe the following precautions:
1. Sealing should be done in a manner that avoids fluid splatter.
2. Always dispose of blood-contaminated products in a manner consistent with established BIOHAZARD safety procedures.
- STORAGE
-
I. BLOOD COLLECTION INSTRUCTIONS FOR SYSTEMS CONTAINING A Y SAMPLING SITE(YSS) ONLY
1. Load blood agitation device or suspend blood bag on donor scale and adjust donor scale to desired collection gross weight as per manufacturer's instructions.
2. Clamp donor tubing between DonorCare® Needle Guard (DCNG) and Y Sampling Site.
3. Secure donor tubing above the Y connector and disinfect site of phlebotomy.
4. If using blood pressure cuff, inflate to not more than 60 mm Hg.
5. Remove donor needle cover and accomplish phlebotomy.
6. Release clamp and ensure there is blood flow. Reduce pressure as required.
7. Slide the DCNG midway over the needle hub and securely tape DCNG to the donor's arm as close to the top of the DCNG as possible. Note: If blood flow is slow, slide DCNG away from the needle hub, adjust and re-engage DCNG. If repeated needle adjustment is necessary, slide DCNG away from the needle hub and re-engage at the end of blood collection.
8. Collect appropriate volume of blood into collection bag, as indicated on packaging.
Note: Mix blood and anticoagulant frequently during collection, for example, once every 45 seconds, and immediately after collection. If blood agitation device is used, follow manufacturer's operating instructions.
9. After required amount of blood has been collected, seal donor tubing close to Y Sampling Site.*
10. For blood sampling, remove the Y Sampling Site needle cover. Ensure the protective sheath is in place over the sampling needle.
11. Fasten the vacuum tube holder on to the base of the sampling needle.
12. Collect blood samples into vacuum tubes.
13. Ensure the vacuum tubes are centered within the vacuum tube holder during sample collection.
14. Maintain forward pressure on the vacuum tubes during sample collection. Note: After the last tube is collected, it is recommended that the vacuum tube holder be left in place.
15. After blood samples are collected, clamp donor tubing between the Y Sampling Site and DCNG, and as close to the DCNG as possible.
16. Release any remaining pressure from the donor's arm.
17. DCNG must be held stationary while the needle is withdrawn into it. While holding sides of DCNG near the front, grasp the tubing below the clamp and pull the needle into the DCNG until it locks into place, and the needle hub engages the bottom of the DCNG.
18. Insert the DCNG into the vacuum tube holder. Note: It is recommended that the DCNG be inserted securely into the vacuum tube holder, prior to discarding.
19. Seal donor tubing adjacent to DCNG.* Detach and discard needle, DCNG,Y Sampling Site and tubing.*
20. Strip tubing between seal and collection bag.
21. Continue to “Processing Instructions”, Section IV, Step 1.
-
II. BLOOD COLLECTION INSTRUCTION FOR SYSTEMS CONTAINING A SAMPLE DIVERSION POUCH WITH OR WITHOUT A PRE-ATTACHED SAMPLOK® VACUUM TUBE HOLDER
When using systems with a pre-attached SampLok vacuum tube holder, follow instructions as noted below, but refer to Section III when indicated to do so.
1. Load blood agitation device or suspend blood bag on donor scale and adjust donor scale to desired collection gross weight as per manufacturer's instructions.
2. Clamp donor tubing between DonorCare Needle Guard (DCNG) and Sampling Site.
3. Secure donor tubing above the Y connector and disinfect site of phlebotomy.
4. If using blood pressure cuff, inflate to not more than 60 mm Hg.
5. Remove donor needle cover and accomplish phlebotomy.
6. Release clamp and ensure there is blood flow.
7. Slide the DCNG midway over the needle hub and securely tape DCNG to the donor's arm as close to the top of the DCNG as possible. Note: If blood flow is slow, slide DCNG away from the needle hub, adjust and re-engage DCNG. If repeated needle adjustment is necessary, slide DCNG away from the needle hub and re-engage at the end of blood collection.
8. The donor blood will be automatically diverted to the sample diversion pouch. Once the sample diversion pouch is filled, close clamp immediately on tubing between the sample diversion pouch and Y connector. Warning: To avoid risk of air embolism to donor, do not squeeze sample diversion pouch while tubing is open.
9. Open snap-open closure between the Y connector and the collection bag to initiate blood collection. Reduce pressure as needed.
10. Permanently seal tubing between the sample diversion pouch and the Y connector to maintain sterility of the system prior to collecting blood samples.* Note: When using systems with a pre-attached SampLok vacuum tube holder, go to Section III.
11. For blood sampling, remove the Sampling Site needle cover. Ensure the protective sheath is in place over the sampling needle.
12. Fasten the vacuum tube holder on to the base of the sampling needle.
13. Position the sample diversion pouch downwards so that the air rises to the top of the pouch and away from the vacuum tube holder. Note: Drawing air into the vacuum tube may cause hemolysis.
14. Collect blood samples from the sample diversion pouch into vacuum tubes within approximately four minutes to avoid possible clot formation.
15. Ensure the vacuum tubes are centered within the vacuum tube holder during sample collection.
16. Maintain forward pressure on the vacuum tubes during sample collection. Note: After the last tube is collected, it is recommended that the vacuum tube holder be left in place.
17. Collect appropriate volume of blood into collection bag as indicated on packaging. Note: Mix blood and anticoagulant frequently during collection, for example, once every 45 seconds, and immediately after collection. If blood agitation device is used, follow manufacturer's operating instructions.
18. After required amount of blood has been collected, seal donor tubing close to snap-open closure.*
19. Clamp donor tubing between the Y connector and DCNG, as close as possible to the DCNG.
20. Release any remaining pressure from donor's arm.
21. DCNG must be held stationary while the needle is withdrawn into it. While holding sides of DCNG near the front, grasp the tubing below the clamp and pull the needle into the DCNG until it locks into place, and the needle hub engages the bottom of the DCNG.
22. Insert the DCNG into the vacuum tube holder, if desired. Note: It is recommended that the DCNG be inserted securely into the vacuum tube holder, prior to discarding.
23. Seal donor tubing adjacent to DCNG.* Detach and discard needle, DCNG, sample diversion pouch and tubing.*
24. Strip tubing between seal and collection bag.
25. Continue to “Processing Instructions”, Section IV, Step 1.
-
III. WHEN USING SYSTEMS WITH A PRE-ATTACHED SAMPLOK® VACUUM TUBE HOLDER
1. To collect blood samples, open lid from SampLok vacuum tube holder.
2. Open snap-open closure between sample diversion pouch and SampLok vacuum tube holder.
3. Position the sample diversion pouch downwards so that the air rises to the top of the pouch and away from the SampLok vacuum tube holder. Note: Drawing air into the vacuum tube may cause hemolysis.
4. Collect blood samples from the sample diversion pouch into vacuum tubes within approximately four minutes to avoid possible clot formation.
5. Ensure the vacuum tubes are centered within the SampLok vacuum tube holder during sample collection.
6. Maintain forward pressure on the vacuum tubes during sample collection.
7. The lid may be closed on the SampLok vacuum tube holder after sample collection.
8. Return to Section II, Step 17. Note: When collection of unit is complete, and the donor needle is engaged in the DCNG, open the lid of the SampLok vacuum tube holder and insert the DCNG into the holder. Twist until it locks into place. An audible click will confirm that it is locked
-
IV. PROCESSING INSTRUCTIONS
1. Process whole blood within 72 hours of collection.
2. Mix whole blood/anticoagulant thoroughly.
3. Load whole blood and satellite bags into centrifuge cup, ensuring that the tubing stays in the top half of the cup.
4. Centrifuge at appropriate conditions to produce desired components.
5. Carefully remove the unit from the centrifuge and place the red cell storage bag in the plasma expressor.
6. Clamp tubing to extra satellite bags, if present.
7. Gently apply expressor pressure.
8. Open snap-open closure to satellite bag and express plasma.
9. After plasma is expressed, clamp tubing between red cell storage bag and Y connector, and release expressor pressure.
10. Clamp tubing between the Y connector and plasma bag.
11. Hang AS-3 bag above red cell storage bag and remove clamp from tubing to the red cell storage bag.
12. Open snap-open closure on the bag containing the AS-3 additive solution and transfer to the bag containing the red cells. Note: AS-3 solution should be added to the packed red blood cells immediately after removal of plasma.
Transfer AS-3 solution under one of the following processing conditions:
a. within 8 hours of collection if whole blood is held at room temperature.
b. within 72 hours of collection if whole blood is refrigerated.
13. Seal tubing and detach the bag containing packed red cells, and set aside plasma for further processing.*
14. Gently mix packed red cells and AS-3 solution.
15. Store CP2D/AS-3 preserved red blood cells at 1—6 °C for up to 42 days and use as indicated. Note: If AS-3 is not used, whole blood or red blood cells in CP2D alone may be stored at 1—6 °C for up to 21 days.
- HOW SUPPLIED
-
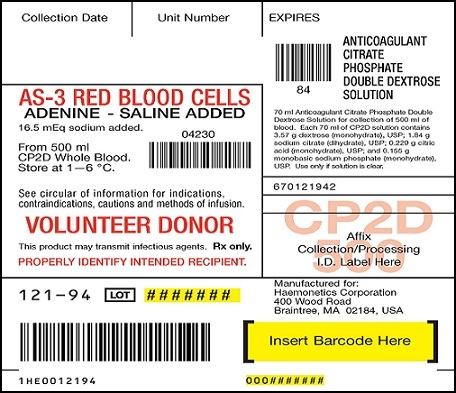
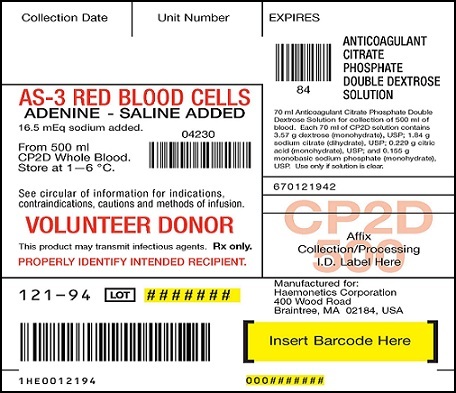
PRINCIPAL DISPLAY PANEL
AS-3 RED BLOOD CELLS ADENINE-SALINE ADDED 16.5 mEq sodium added
From 500ml CP2D Whole Blood Store at 1—6 °C
Anticoagulant Citrate Phosphate Double Dextrose Solution
70ml Anticoagulant Citrate Phosphate Double Dextrose Solution for collection of 500ml of blood. Each 70ml of CP2D solution contains 3.57g dextrose (monohydrate), USP; 1.84g sodium citrate (dihydrate), USP; 0.229g citric acid (monohydrate), USP; and 0.155g monobasic sodium phosphate (monohydrate), USP. Use only if solution is clear.
See circular of information for indications, contraindications, cautions and methods of infusion. VOLUNTEER DONOR. This product may transmit infectious agents. Rx only. PROPERLY IDENTIFY INTENDED RECIPIENT.
Red cell storage in CLX bag limited to 21 days. Store platelets 5 days at 20—24°C
See circular of information for indications, contraindications, cautions and methods of infusion. VOLUNTEER DONOR. This product may transmit infectious agents. Rx only. PROPERLY IDENTIFY INTENDED RECIPIENT.
110ml of preservative AS-3 solution contains 1.21g dextrose (monohydrate), USP; 0.647 g sodium chloride (dihydrate), USP; 0.451 g sodium chloride USP; 0.304 g monobasic sodium phosphate (monohydrate), USP; and 0.033g adenine, USP. Contains 16.5 mEq of sodium.
Use only if solution is clear.
See circular of information for indications, contraindications, cautions and methods of infusion. VOLUNTEER DONOR. This product may transmit infectious agents. Rx only. PROPERLY IDENTIFY INTENDED RECIPIENT.
BLOOD COLLECTION SYSTEM with Nutricel Additive Solution
CP2D/AS-3 Quadruple with Sample Diversion Pouch
For collection of 500ml of blood and preparation of red blood cells, plasma and platelets
Each unit consists of a collection bag with 70ml of CP2D solution, an additive bag with 110ml of AS-3 solution, and two empty CLX satellite bags. Each 70ml of CP2D solution contains 3.57 g dextrose (monohydrate), USP; 1.84g sodium citrate (dihydrate), USP; 0.229 g citric acid (monohydrate), USP; and 0.155g monobasic sodium phosphate (monohydrate), USP. Each 110ml of AS-3 solution contains 1.21 g dextrose (monohydrate), USP; 0.647 g sodium citrate (dihydrate), USP; 0.451 g sodium chloride, USP; 0.304 g monobasic sodium phosphate (monohydrate), USP; 0.046 g citric acid (monohydrate), USP; and 0.033 g adenine, USP.
Sterile, non pyrogenic fluid path. Sterilized by steam. See accompanying directions for use. Rx only. Store at room temperature. Unused bags in opened pouches may be kept 30 days by folding and SECURING open end of pouch to prevent possible loss of moisture.
- REFERENCES
- MANUFACTURER
-
INGREDIENTS AND APPEARANCE
NUTRICEL ADDITIVE SOLUTION - CP2D
cp2d/as-3 anticoagulant and additive system solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53157-100 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 3.57 g in 70 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) Product Characteristics Color yellow (yellowish solution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53157-100-96 8 in 1 BOX 1 NDC:53157-100-95 3 in 1 POUCH 1 NDC:53157-100-94 70 mL in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN820915 09/13/2013 NUTRICEL ADDITIVE SOLUTION - AS-3
cp2d/as-3 anticoagulant and additive system solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53157-101 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 1.21 g in 110 mL ADENINE (UNII: JAC85A2161) (ADENINE - UNII:JAC85A2161) ADENINE 0.033 g in 110 mL Inactive Ingredients Ingredient Name Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (yellowish solution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53157-101-99 8 in 1 BOX 1 NDC:53157-101-98 3 in 1 POUCH 1 NDC:53157-101-97 110 mL in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN820915 09/13/2013 Labeler - Haemonetics Manufacturing Inc. (078598396) Registrant - Haemonetics Manufacturing Inc. (078598396) Establishment Name Address ID/FEI Business Operations Haemonetics Manufacturing Inc. 078598396 manufacture(53157-100)