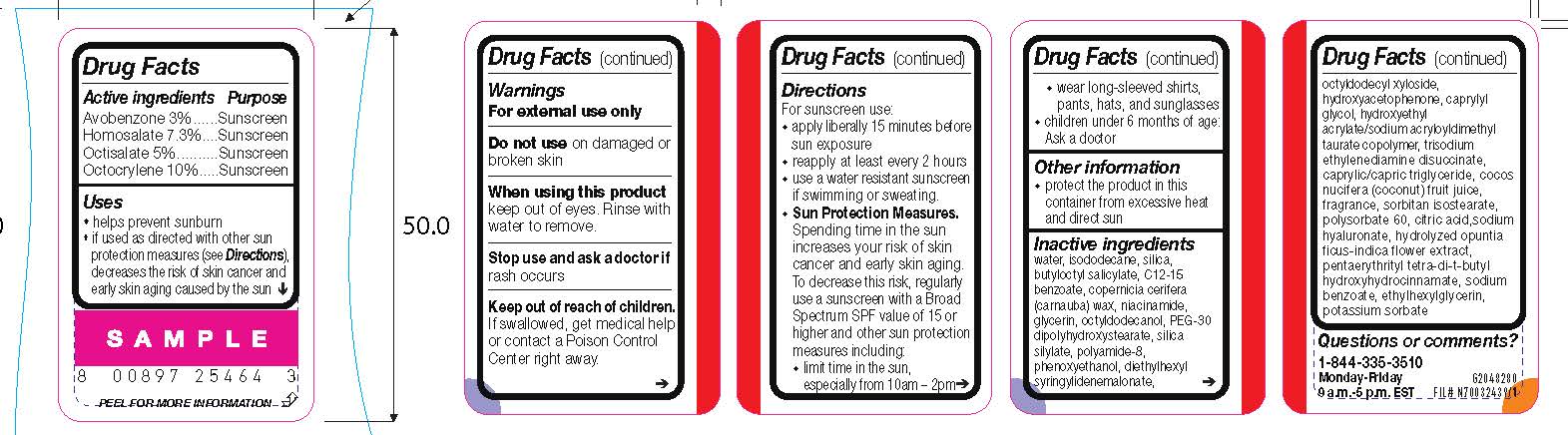
Label: NYX PROFESSIONAL MAKEUP BLURSCREEN PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN- avobenzone, homosalate, octisalate and octocrylene lotion
- NDC Code(s): 49967-990-01, 49967-990-02
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
● apply liberally 15 minutes before sun exposure
● reapply at least every 2 hours
● use a water resistant sunscreen if swimming or sweating
● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
● limit time in the sun, especially from 10 a.m. – 2 p.m.
● wear long-sleeved shirts, pants, hats, and sunglasses
● children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, isododecane, silica, butyloctyl salicylate, c12-15 alkyl benzoate, copernicia cerifera (carnauba) wax, niacinamide, glycerin, octyldodecanol, PEG-30 dipolyhydroxystearate, silica silylate, polyamide-8, phenoxyethanol, diethylhexyl syringylidenemalonate, octyldodecyl xyloside, hydroxyacetophenone, caprylyl glycol, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, trisodium ethylenediamine disuccinate, caprylic/capric triglyceride, cocos nucifera (coconut) fruit juice, fragrance, sorbitan isostearate, polysorbate 60, citric acid, sodium hyaluronate, hydrolyzed opuntia ficus-indica flower extract, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, sodium benzoate, ethylhexylglycerin, potassium sorbate
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NYX PROFESSIONAL MAKEUP BLURSCREEN PRIMER BROAD SPECTRUM SPF 30 SUNSCREEN
avobenzone, homosalate, octisalate and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-990 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 73 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CARNAUBA WAX (UNII: R12CBM0EIZ) NIACINAMIDE (UNII: 25X51I8RD4) GLYCERIN (UNII: PDC6A3C0OX) OCTYLDODECANOL (UNII: 461N1O614Y) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) POLYAMIDE-8 (4500 MW) (UNII: 77723GV81A) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) OCTYLDODECYL XYLOSIDE (UNII: 8Z6VNR46QM) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCONUT JUICE (UNII: AMN6S4M09G) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) POLYSORBATE 60 (UNII: CAL22UVI4M) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) SODIUM BENZOATE (UNII: OJ245FE5EU) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-990-01 30 mL in 1 TUBE; Type 0: Not a Combination Product 01/01/2024 2 NDC:49967-990-02 2 mL in 1 PACKAGE; Type 0: Not a Combination Product 04/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2024 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations Accupac, LLC 061595175 manufacture(49967-990) , pack(49967-990)