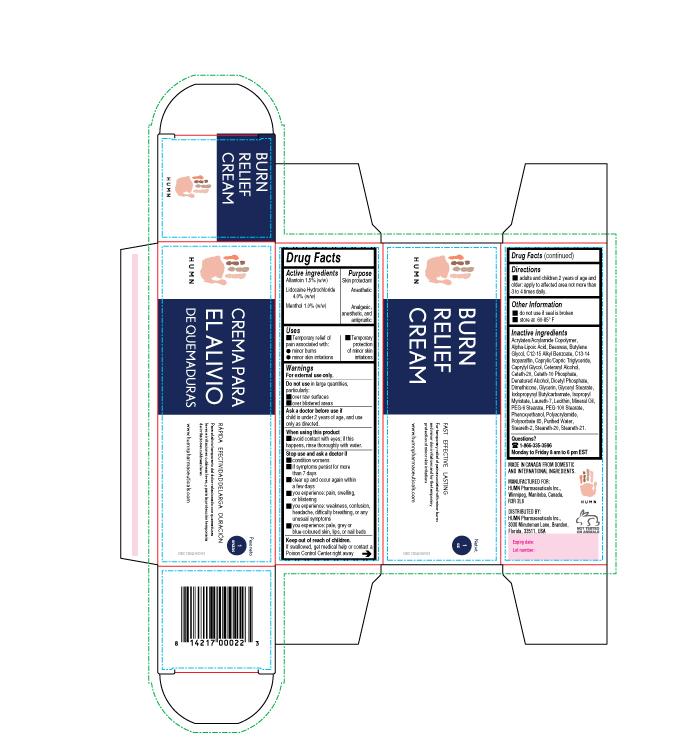
Label: BURN RELIEF- allantoin, lidocaine, menthol cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 72042-003-03 - Packager: HUMN Pharmaceuticals Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 19, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
-
STOP USE
Stop use and ask a doctor
• condition worsens • if symptoms persist for more than 7 days
• clear up and occur again within a few days • you experience: pain, swelling, or blistering
• you experience: weakness, confusion, headache, difficulty breathing, or any unusual symptoms
• you experience: pale, grey or blue-coloured skin, lips, or nail beds
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INFORMATION FOR PATIENTS
-
INACTIVE INGREDIENT
Inactive ingredients
Acrylates/Acrylamide Copolymer, Alpha-Lipoic Acid, Beeswax, Butylene Glycol, C12-15 Alkyl Benzoate, C13-14 Isoparaffin, Caprylic/Capric Triglyceride, Caprylyl Glycol, Ceteraryl Alcohol, Ceteth-20, Ceteth-10 Phosphate, Denatured Alcohol, Dicetyl Phosphate, Dimethicone, Glycerin, Glyceryl Stearate, Iodopropynyl Butylcarbamate, Isopropyl Myristate, Laureth-7, Lecithin, Mineral Oil, PEG-6 Stearate, PEG-100 Stearate, Phenoxyethanol, Polyacrylamide, Polysorbate 85, Purified Water, Steareth-2, Steareth-20, Steareth-21.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BURN RELIEF
allantoin, lidocaine, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72042-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 mg in 100 mg LIDOCAINE HYDROCHLORIDE ANHYDROUS (UNII: EC2CNF7XFP) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 mg in 100 mg ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 1.5 mg in 100 mg Inactive Ingredients Ingredient Name Strength CETETH-20 (UNII: I835H2IHHX) ALCOHOL (UNII: 3K9958V90M) ALUMINUM DICETYL PHOSPHATE (UNII: WMV3R5DS7O) GLYCERIN (UNII: PDC6A3C0OX) MINERAL OIL (UNII: T5L8T28FGP) PEG-6 STEARATE (UNII: 8LQC57C6B0) PEG-100 STEARATE (UNII: YD01N1999R) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) STEARETH-2 (UNII: V56DFE46J5) STEARETH-20 (UNII: L0Q8IK9E08) CETETH-10 PHOSPHATE (UNII: 4E05O5N49G) PEG-120 GLYCERYL STEARATE (UNII: 6941286E4I) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LAURETH-7 (UNII: Z95S6G8201) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE 100 (UNII: RO266O364U) POLYSORBATE 85 (UNII: A7F3N56197) WATER (UNII: 059QF0KO0R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) ACRYLAMIDE (UNII: 20R035KLCI) CETYL ALCOHOL (UNII: 936JST6JCN) YELLOW WAX (UNII: 2ZA36H0S2V) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-LIPOIC ACID (UNII: 73Y7P0K73Y) STEARETH-21 (UNII: 53J3F32P58) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72042-003-03 1 in 1 CARTON 05/19/2018 1 28300 mg in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/16/2018 Labeler - HUMN Pharmaceuticals Inc (245630272) Registrant - Delta Pharma Inc (200161730) Establishment Name Address ID/FEI Business Operations Delta Pharma Inc. 200161730 manufacture(72042-003)