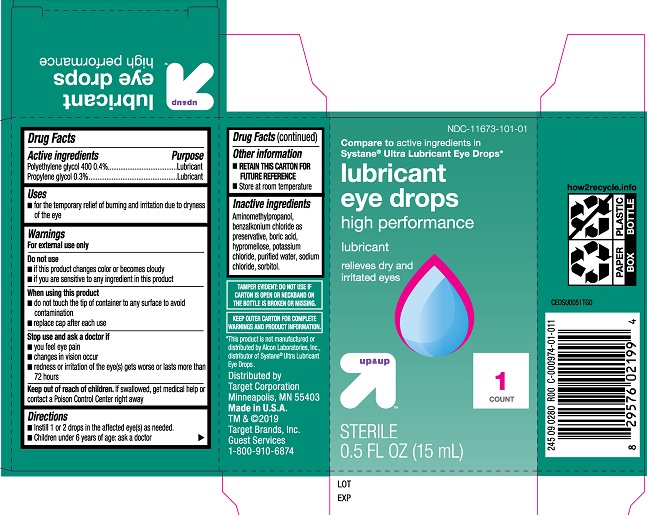
Label: TARGET LUBRICANT EYE DROPS HIGH PERFORMANCE- polyethylene glycol 400, propylene glycol solution/ drops
- NDC Code(s): 11673-101-01
- Packager: Target Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- box
-
INGREDIENTS AND APPEARANCE
TARGET LUBRICANT EYE DROPS HIGH PERFORMANCE
polyethylene glycol 400, propylene glycol solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-101 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) (POLYETHYLENE GLYCOL 400 - UNII:B697894SGQ) POLYETHYLENE GLYCOL 400 0.4 g in 100 mL PROPYLENE GLYCOL (UNII: 6DC9Q167V3) (PROPYLENE GLYCOL - UNII:6DC9Q167V3) PROPYLENE GLYCOL 0.3 g in 100 mL Inactive Ingredients Ingredient Name Strength AMINOMETHYLPROPANOL (UNII: LU49E6626Q) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BORIC ACID (UNII: R57ZHV85D4) HYPROMELLOSES (UNII: 3NXW29V3WO) POTASSIUM CHLORIDE (UNII: 660YQ98I10) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITOL (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-101-01 1 in 1 BOX 02/11/2019 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 02/11/2019 Labeler - Target Corporation (006961700) Registrant - KC Pharmaceuticals, Inc. (174450460) Establishment Name Address ID/FEI Business Operations KC Pharmaceuticals, Inc. 174450460 manufacture(11673-101) , pack(11673-101) , label(11673-101)