Label: CHILDRENS ROBITUSSIN 12 HOUR COUGH RELIEF- dextromethorphan polistirex suspension, extended release
- NDC Code(s): 0031-8725-10, 0031-8726-10
- Packager: Haleon US Holdings LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not useif you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- chronic cough that lasts as occurs with smoking, asthma, or emphysema
- cough that occurs with too much phlegm (mucus)
-
DOSAGE & ADMINISTRATION
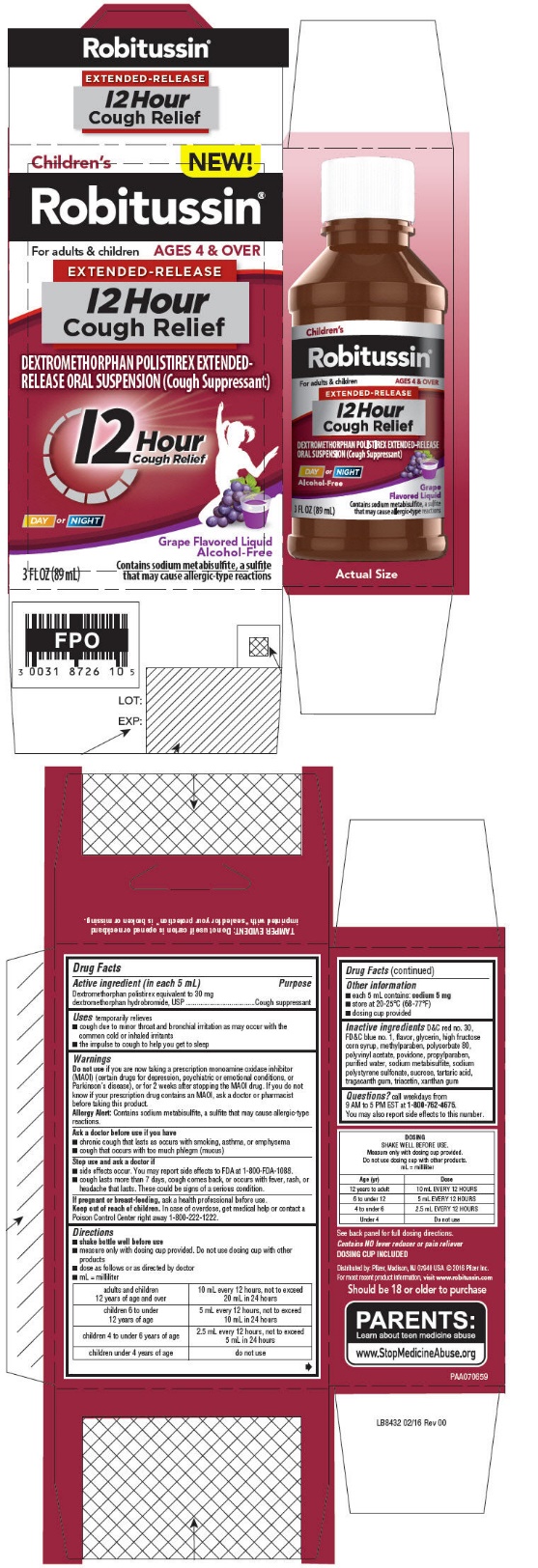
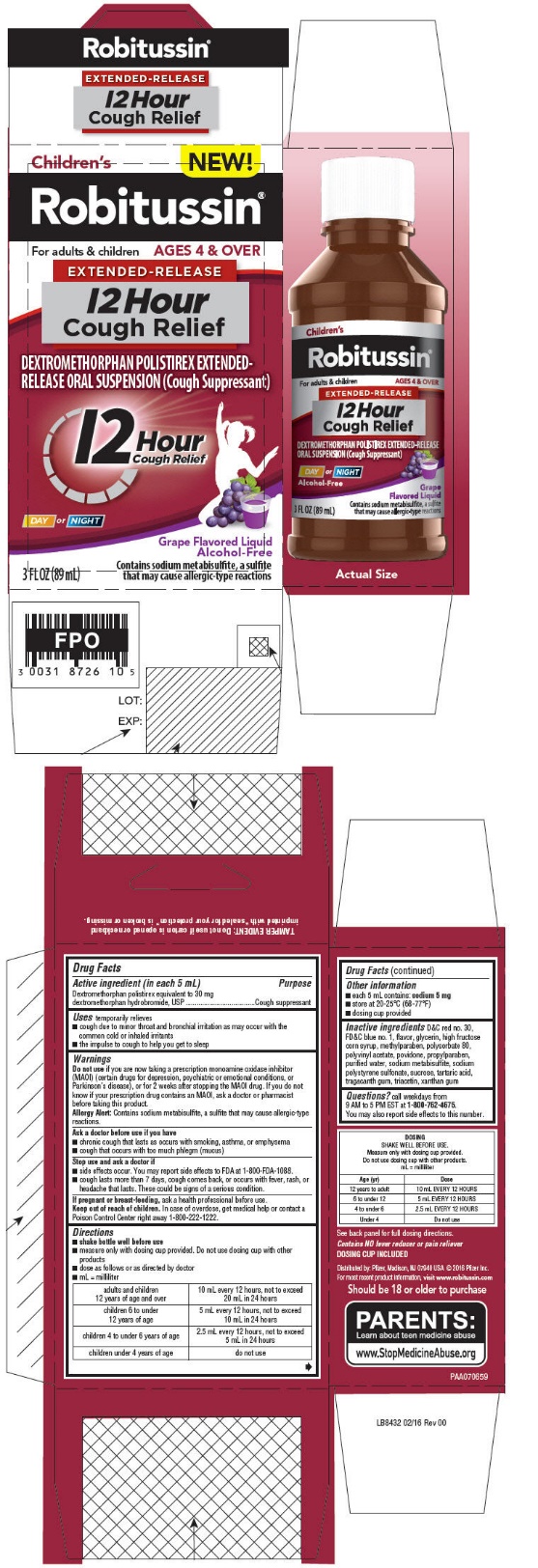
Directions
- shake bottle well before use
- measure only with dosing cup provided. Do not use dosing cup with other products
- dose as follows or as directed by doctor
- mL = milliliter
adults and children 12 years of age and over
10 mL every 12 hours, not to exceed 20 mL in 24 hours
children 6 to under 12 years of age
5 mL every 12 hours, not to exceed 10 mL in 24 hours
children 4 to under 6 years of age
2.5 mL every 12 hours, not to exceed 5 mL in 24 hours
children under 4 years of age
do not use
- STORAGE AND HANDLING
- Inactive ingredients (Grape flavor)
- Inactive ingredients (Orange flavor)
- QUESTIONS
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 89 mL Bottle Carton - Orange
NEW!
Children's
Robitussin ®For adults & children
AGES 4 & OVEREXTENDED-RELEASE
12 Hour
Cough ReliefDEXTROMETHORPHAN POLISTIREX EXTENDED-
RELEASE ORAL SUSPENSION (Cough Suppressant)12 Hour
Cough ReliefDAY or NIGHT
Orange Flavored Liquid
Alcohol-Free3 FL OZ (89 mL)
Contains sodium metabisulfite, a sulfite
that may cause allergic-type reactions -
PRINCIPAL DISPLAY PANEL - 89 mL Bottle Carton - Grape
NEW!
Children's
Robitussin ®For adults & children
AGES 4 & OVEREXTENDED-RELEASE
12 Hour
Cough ReliefDEXTROMETHORPHAN POLISTIREX EXTENDED-
RELEASE ORAL SUSPENSION (Cough Suppressant)12 Hour
Cough ReliefDAY or NIGHT
Grape Flavored Liquid
Alcohol-Free3 FL OZ (89 mL)
Contains sodium metabisulfite, a sulfite
that may cause allergic-type reactions -
INGREDIENTS AND APPEARANCE
CHILDRENS ROBITUSSIN 12 HOUR COUGH RELIEF
dextromethorphan polistirex suspension, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0031-8725 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength POLISTIREX (UNII: 5H9W9GTW27) D&C RED NO. 30 (UNII: 2S42T2808B) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ACETATE (UNII: 32K497ZK2U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) SUCROSE (UNII: C151H8M554) TARTARIC ACID (UNII: W4888I119H) TRAGACANTH (UNII: 2944357O2O) TRIACETIN (UNII: XHX3C3X673) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color orange Score Shape Size Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0031-8725-10 1 in 1 CARTON 07/05/2016 1 89 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091135 07/05/2016 CHILDRENS ROBITUSSIN 12 HOUR COUGH RELIEF
dextromethorphan polistirex suspension, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0031-8726 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength POLISTIREX (UNII: 5H9W9GTW27) D&C RED NO. 30 (UNII: 2S42T2808B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ACETATE (UNII: 32K497ZK2U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) SUCROSE (UNII: C151H8M554) TARTARIC ACID (UNII: W4888I119H) TRAGACANTH (UNII: 2944357O2O) TRIACETIN (UNII: XHX3C3X673) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color purple Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0031-8726-10 1 in 1 CARTON 07/05/2016 1 89 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091135 07/05/2016 Labeler - Haleon US Holdings LLC (079944263)