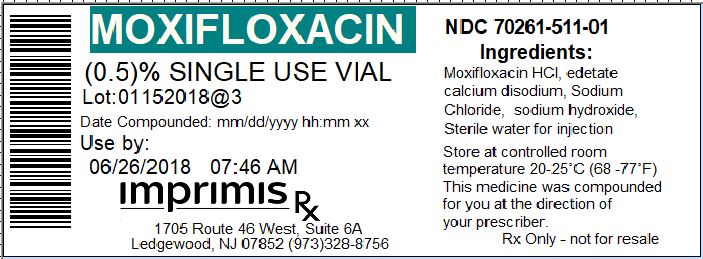
Label: MOXIFLOXACIN PF injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 70261-511-01 - Packager: ImprimisRx NJ
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 8, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STORAGE AND HANDLING
- Vial Label
-
INGREDIENTS AND APPEARANCE
MOXIFLOXACIN PF
moxifloxacin pf injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70261-511 Route of Administration INTRAOCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIFLOXACIN HYDROCHLORIDE MONOHYDRATE (UNII: B8956S8609) (MOXIFLOXACIN - UNII:U188XYD42P) MOXIFLOXACIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 8 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70261-511-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 01/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2018 Labeler - ImprimisRx NJ (931390178) Registrant - ImprimisRx NJ (931390178) Establishment Name Address ID/FEI Business Operations Imprimis Pharmaceuticals 080431967 manufacture(70261-511)