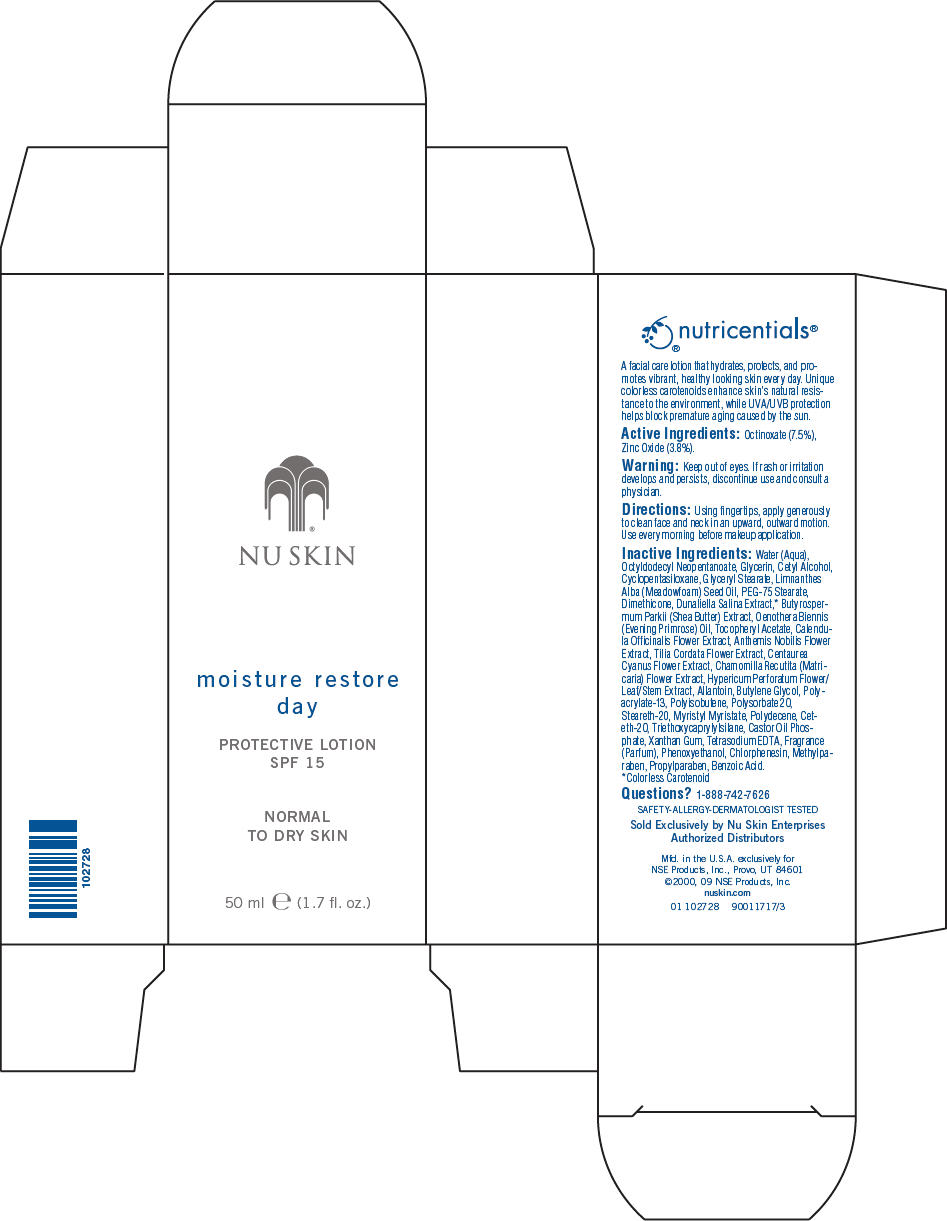
Label: NU SKIN MOISTURE RESTORE DAY PROTECTIVE SPF 15 NORMAL TO DRY SKIN- octinoxate and titanium dioxide lotion
- NDC Code(s): 62839-2728-1
- Packager: NSE Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Warning
- Directions
-
Inactive Ingredients
Water (Aqua), Octyldodecyl Neopentanoate, Glycerin, Cetyl Alcohol, Cyclopentasiloxane, Glyceryl Stearate, Limnanthes Alba (Meadowfoam) Seed Oil, PEG-75 Stearate, Dimethicone, Dunaliella Salina Extract,1 Butyrospermum Parkii (Shea Butter) Extract, Oenothera Biennis (Evening Primrose) Oil, Tocopheryl Acetate, Calendula Officinalis Flower Extract, Anthemis Nobilis Flower Extract, Tilia Cordata Flower Extract, Centaurea Cyanus Flower Extract, Chamomilla Recutita (Matricaria) Flower Extract, Hypericum Perforatum Flower/Leaf/Stem Extract, Allantoin, Butylene Glycol, Polyacrylate-13, Polyisobutene, Polysorbate 20, Steareth-20, Myristyl Myristate, Polydecene, Ceteth-20, Triethoxycaprylylsilane, Castor Oil Phosphate, Xanthan Gum, Tetrasodium EDTA, Fragrance (Parfum), Phenoxyethanol, Chlorphenesin, Methylparaben, Propylparaben, Benzoic Acid.
- 1
- Colorless Carotenoid
- Questions?
- PRINCIPAL DISPLAY PANEL - 50 ml Carton
-
INGREDIENTS AND APPEARANCE
NU SKIN MOISTURE RESTORE DAY PROTECTIVE SPF 15 NORMAL TO DRY SKIN
octinoxate and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-2728 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 38 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Octyldodecyl Neopentanoate (UNII: X8725R883T) Glycerin (UNII: PDC6A3C0OX) Cetyl Alcohol (UNII: 936JST6JCN) Cyclomethicone 5 (UNII: 0THT5PCI0R) Glyceryl Monostearate (UNII: 230OU9XXE4) Meadowfoam Seed Oil (UNII: 412ZHA4T4Y) PEG-75 Stearate (UNII: OT38R0N74H) Dimethicone (UNII: 92RU3N3Y1O) Sheanut Oil (UNII: O88E196QRF) Dunaliella Salina (UNII: F4O1DKI9A6) Evening Primrose Oil (UNII: 3Q9L08K71N) Calendula Officinalis Flower (UNII: P0M7O4Y7YD) Chamaemelum Nobile Flower (UNII: O2T154T6OG) Tilia Cordata Flower (UNII: CFN6G1F6YK) Centaurea Cyanus Flower (UNII: QZ239038YC) Chamomile (UNII: FGL3685T2X) St. John's Wort (UNII: UFH8805FKA) Allantoin (UNII: 344S277G0Z) Butylene Glycol (UNII: 3XUS85K0RA) Polysorbate 20 (UNII: 7T1F30V5YH) Steareth-20 (UNII: L0Q8IK9E08) Myristyl Myristate (UNII: 4042ZC00DY) Ceteth-20 (UNII: I835H2IHHX) Triethoxycaprylylsilane (UNII: LDC331P08E) Xanthan Gum (UNII: TTV12P4NEE) Edetate Sodium (UNII: MP1J8420LU) Phenoxyethanol (UNII: HIE492ZZ3T) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Benzoic Acid (UNII: 8SKN0B0MIM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-2728-1 1 in 1 CARTON 06/10/2001 1 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 06/10/2001 Labeler - NSE Products, Inc. (803486393)