Label: COLLAGENASE SANTYL ointment
- NDC Code(s): 50484-010-30, 50484-010-90
- Packager: SMITH & NEPHEW, INC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 17, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
CLINICAL PHARMACOLOGY
Since collagen accounts for 75% of the dry weight of skin tissue, the ability of collagenase to digest collagen in the physiological pH and temperature range makes it particularly effective in the removal of detritus. 1 Collagenase thus contributes towards the formation of granulation tissue and subsequent epithelization of dermal ulcers and severely burned areas. 2, 3, 4, 5, 6 Collagen in healthy tissue or in newly formed granulation tissue is not attacked. 2, 3, 4, 5, 6, 7, 8 There is no information available on collagenase absorption through skin or its concentration in body fluids associated with therapeutic and/or toxic effects, degree of binding to plasma proteins, degree of uptake by a particular organ or in the fetus, and passage across the blood brain barrier.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
The optimal pH range of collagenase is 6 to 8. Higher or lower pH conditions will decrease the enzyme’s activity and appropriate precautions should be taken. The enzymatic activity is also adversely affected by certain detergents, and heavy metal ions such as mercury and silver which are used in some antiseptics. When it is suspected such materials have been used, the site should be carefully cleansed by repeated washings with normal saline before Collagenase Santyl◊ Ointment is applied. Soaks containing metal ions or acidic solutions should be avoided because of the metal ion and low pH. Cleansing materials such as Dakin’s solution and normal saline are compatible with Collagenase Santyl◊ Ointment.
Debilitated patients should be closely monitored for systemic bacterial infections because of the theoretical possibility that debriding enzymes may increase the risk of bacteremia.
A slight transient erythema has been noted occasionally in the surrounding tissue, particularly when Collagenase Santyl◊ Ointment was not confined to the wound. Therefore, the ointment should be applied carefully within the area of the wound. Safety and effectiveness in pediatric patients have not been established.
- ADVERSE REACTIONS
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Collagenase Santyl◊ Ointment should be applied once daily (or more frequently if the dressing becomes soiled, as from incontinence). When clinically indicated, crosshatching thick eschar with a #10 blade allows Collagenase Santyl◊ Ointment more surface contact with necrotic debris. It is also desirable to remove, with forceps and scissors, as much loosened detritus as can be done readily. Use Collagenase Santyl◊ Ointment in the following manner:
1 - Prior to application the wound should be cleansed of debris and digested material by gently rubbing with a gauze pad saturated with normal saline solution, or with the desired cleansing agent compatible with Collagenase Santyl◊ Ointment (See PRECAUTIONS ), followed by a normal saline solution rinse.
2 - Whenever infection is present, it is desirable to use an appropriate topical antibiotic powder. The antibiotic should be applied to the wound prior to the application of Collagenase Santyl◊ Ointment. Should the infection not respond, therapy with Collagenase Santyl◊ Ointment should be discontinued until remission of the infection.
3 - Collagenase Santyl◊ Ointment may be applied directly to the wound or to a sterile gauze pad which is then applied to the wound and properly secured.
4 - Use of Collagenase Santyl◊ Ointment should be terminated when debridement of necrotic tissue is complete and granulation tissue is well established.
-
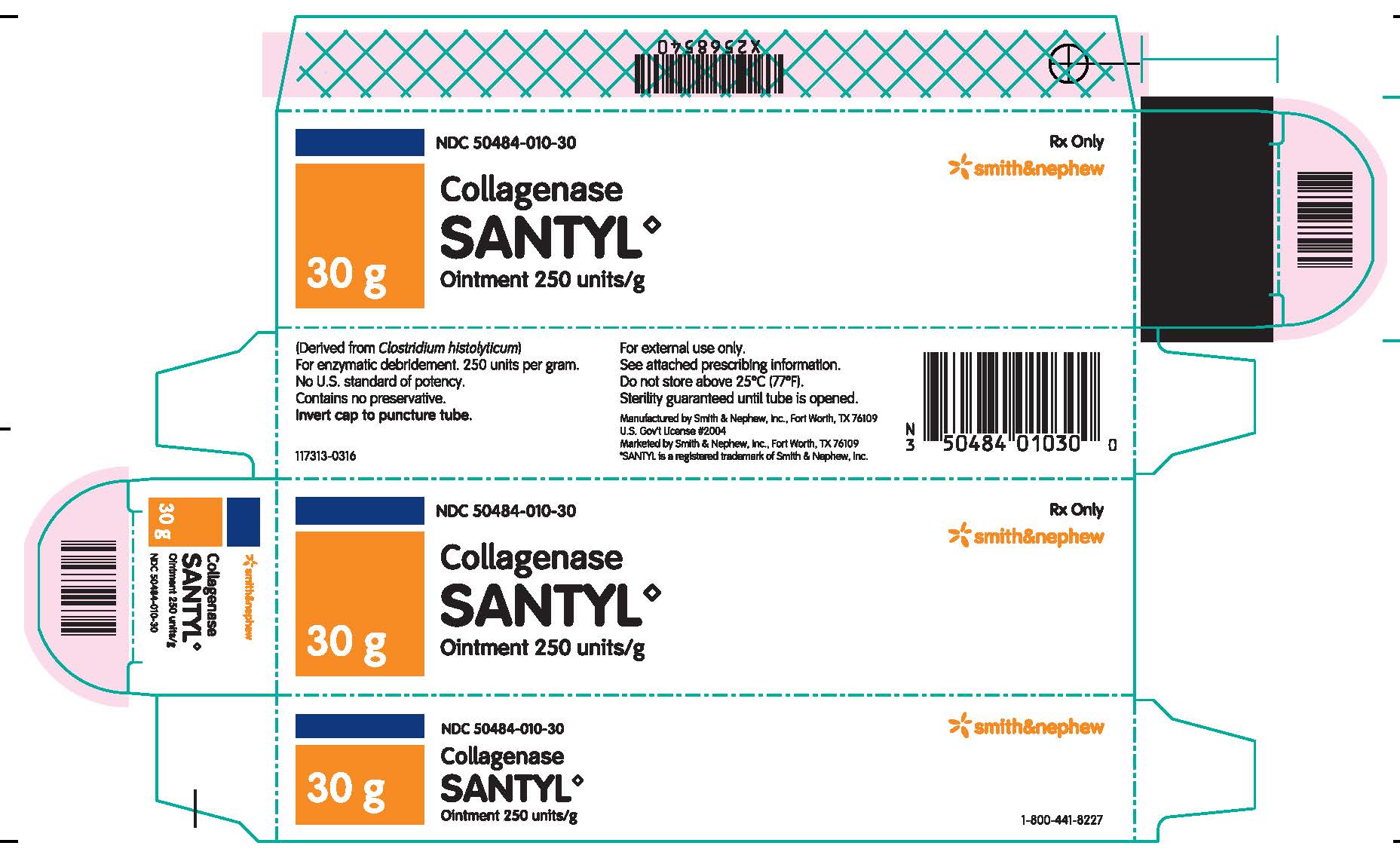
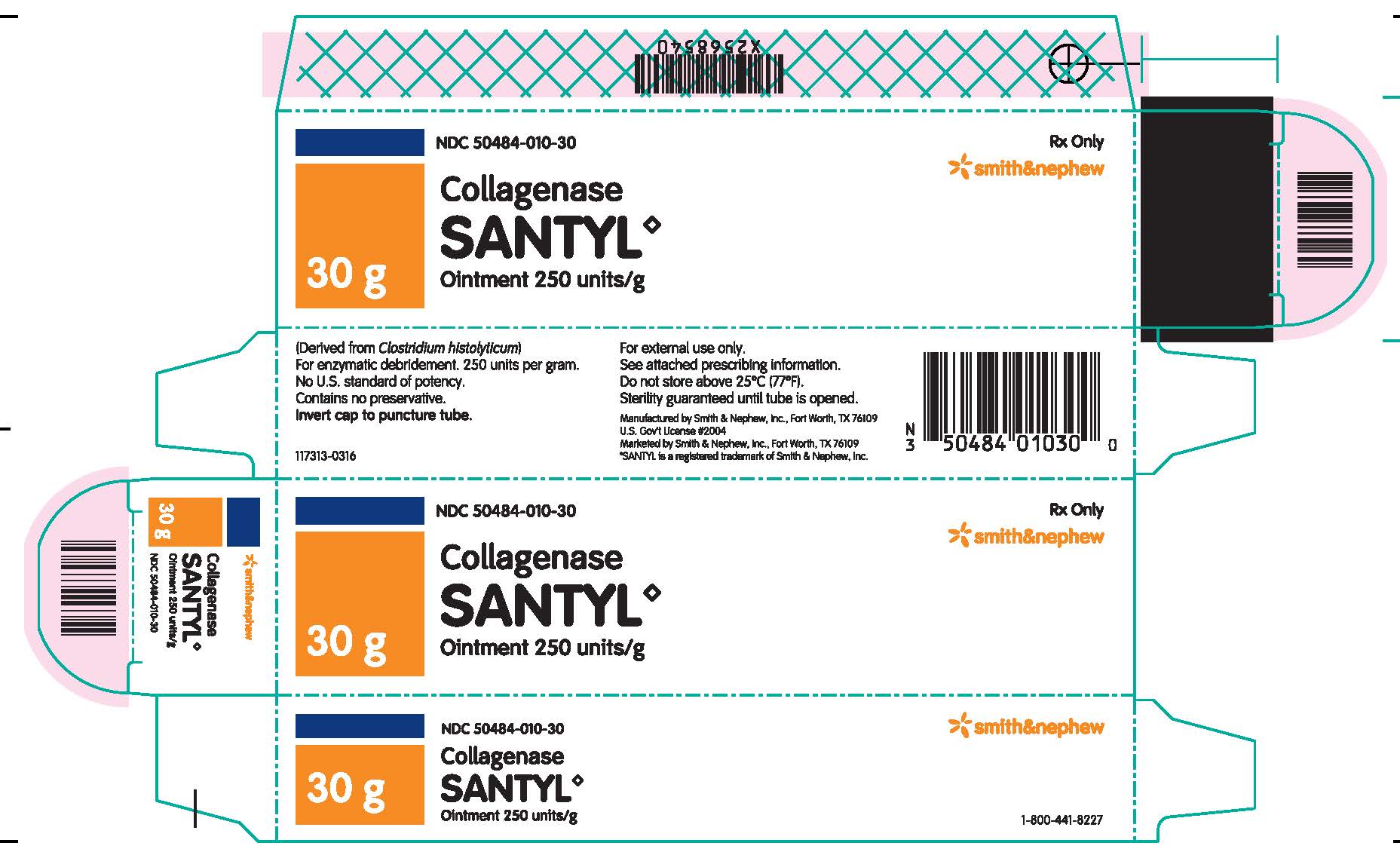
HOW SUPPLIED
Collagenase Santyl◊ Ointment contains 250 units of collagenase enzyme per gram of white petrolatum USP.
Do not store above 25°C (77°F). Sterility guaranteed until tube is opened.
Collagenase Santyl◊ Ointment is available in the following sizes:
30 g tube NDC 50484-010-30
90 g tube NDC 50484-010-90
REFERENCES
1 – Mandl, I., Adv Enzymol. 23:163, 1961.
2 – Boxer, A.M., Gottesman, N., Bernstein, H., & Mandl, I., Geriatrics. 24:75, 1969.
3 – Mazurek, I., Med. Welt. 22:150, 1971.
4 – Zimmermann, WE., in “Collagenase,” Mandl, I., ed., Gordon & Breach, Science Publishers, New York, 1971, p. 131, p. 185.
5 – Vetra, H., & Whittaker, D., Geriatrics. 30:53, 1975.
6 – Rao, D.B., Sane, P.G., & Georgiev, E.L., J. Am. Geriatrics Soc. 23:22, 1975.
7 – Vrabec, R., Moserova, J., Konickova, Z., Behounkova, E., & Blaha, J., J. Hyg. Epidemiol. Microbiol. Immunol. 18:496, 1974.
8 – Lippmann, H.I., Arch. Phys. Med. Rehabil. 54:588, 1973.
9 – German, F. M., in “Collagenase,” Mandl, I., ed., Gordon & Breach, Science Publishers, New York, 1971, p. 165.
10 – Haimovici, H. & Strauch, B., in “Collagenase,” Mandl, I., ed., Gordon & Breach, Science Publishers, New York, 1971, p. 177.
11 – Lee, L.K., & Ambrus, J.L., Geriatrics. 30:91, 1975.
12 – Locke, R.K., & Heifitz, N.M., J. Am. Pod. Assoc. 65:242, 1975.
13 – Varma, A.O., Bugatch, E., & German, F.M., Surg. Gynecol. Obstet. 136:281, 1973.
14 – Barrett, D., Jr., & Klibanski, A., Am. J. Nurs. 73:849, 1973.
15 – Bardfeld, L.A., J. Pod. Ed. 1:41, 1970.
16 – Blum, G., Schweiz, Rundschau Med Praxis. 62:820, 1973. Abstr. in Dermatology Digest, Feb. 1974, p. 36.
17 – Zaruba, F., Lettl, A., Brozkova, L., Skrdlantova, H., & Krs, V., J. Hyg. Epidemiol. Microbiol. Immunol. 18:499, 1974.
18 – Altman, M.I., Goldstein, L., & Horwitz, S., J. Am. Pod. Assoc. 68:11, 1978.
19 – Rehn, V.J., Med. Klin. 58:799, 1963.
20 – Krauss, H., Koslowski, L., & Zimmermann, W. E., Langenbecks Arch. Klin. Chir. 303:23, 1963.
21 – Gruenagel, H.H., Med. Klin. 58:442, 1963.
Manufactured by:
SMITH & NEPHEW, INC
Fort Worth, Texas 76109US Gov’t License #2004
Marketed by:
Smith & Nephew◊1-800-441-8227
Smith & Nephew, Inc.,
Fort Worth, Texas 76109© 2016 Smith & Nephew, Inc.
SANTYL is a registered trademark of Smith & Nephew, Inc.
140749-0316
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- TUBE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COLLAGENASE SANTYL
collagenase santyl ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50484-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COLLAGENASE CLOSTRIDIUM HISTOLYTICUM (UNII: 9X7O8V25IT) (COLLAGENASE CLOSTRIDIUM HISTOLYTICUM - UNII:9X7O8V25IT) COLLAGENASE CLOSTRIDIUM HISTOLYTICUM 250 [arb'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color WHITE (WHITE TO OFF WHITE) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50484-010-30 1 in 1 CARTON 10/18/2006 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:50484-010-90 1 in 1 CARTON 03/10/2014 2 90 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101995 10/18/2006 Labeler - SMITH & NEPHEW, INC (827731451) Establishment Name Address ID/FEI Business Operations Renaissance Lakewood LLC 077744035 MANUFACTURE(50484-010) , pack(50484-010) , analysis(50484-010) Establishment Name Address ID/FEI Business Operations HALO PHARMACEUTICALS, INC. 829609168 MANUFACTURE(50484-010) , pack(50484-010) Establishment Name Address ID/FEI Business Operations Smith & Nephew, Inc. 125458849 analysis(50484-010)