Label: TINTED SPF 45- octinoxate, octisalate, zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 10477-2627-1, 10477-2627-2, 10477-2627-3 - Packager: Goodier Cosmetics, LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 5, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Revision Skincare 1.7oz Retail Intellishade®
Intellishade®
SPF 45 Originalanti-aging
tinted moisturizer
for all skin types
NT WT 1.7 OZ / 48 G
Uses
- helps prevent sunburn
- higher SPF gives more sun protection
- for skin highly sensitive to sunburn
Keep this and all drugs out of reach
of children. If swallowed, get medical
help or contact a Poison Control
Center right away.
Inactive ingredients
Water (Aqua), Cyclopentasiloxane, Ethylhexyl Stearate, Sorbitol,
Caprylic/Capric Triglyceride, Glycerin, Polyglyceryl-4 Isostearate,
Cetyl PEG/PPG 10/1 Dimethicone, Hexyl Laurate, Aloe Barbadensis
Leaf Juice, Cetyl Dimethicone, Betula Alba (White Birch) Extract,
Camellia Sinensis (Green Tea) Leaf Extract, Plankton Extract,
Beeswax, Dimethicone, Squalane, Tocopheryl Acetate, Tetrahexyldecyl
Ascorbate, Hydrogenated Castor Oil, Lecithin, Butylene Glycol, Caprylyl
Glycol, Palmitoyl Tripeptide-5, Saccharomyces Cerevisiae Extract,
Ubiquinone, Retinyl Palmitate, Sodium Chloride, Phenoxyethanol,
Chlorphenesin, Triethoxycaprylylsilane, Benzoic Acid, Sorbic Acid,
Titanium Dioxide, Iron Oxides
-
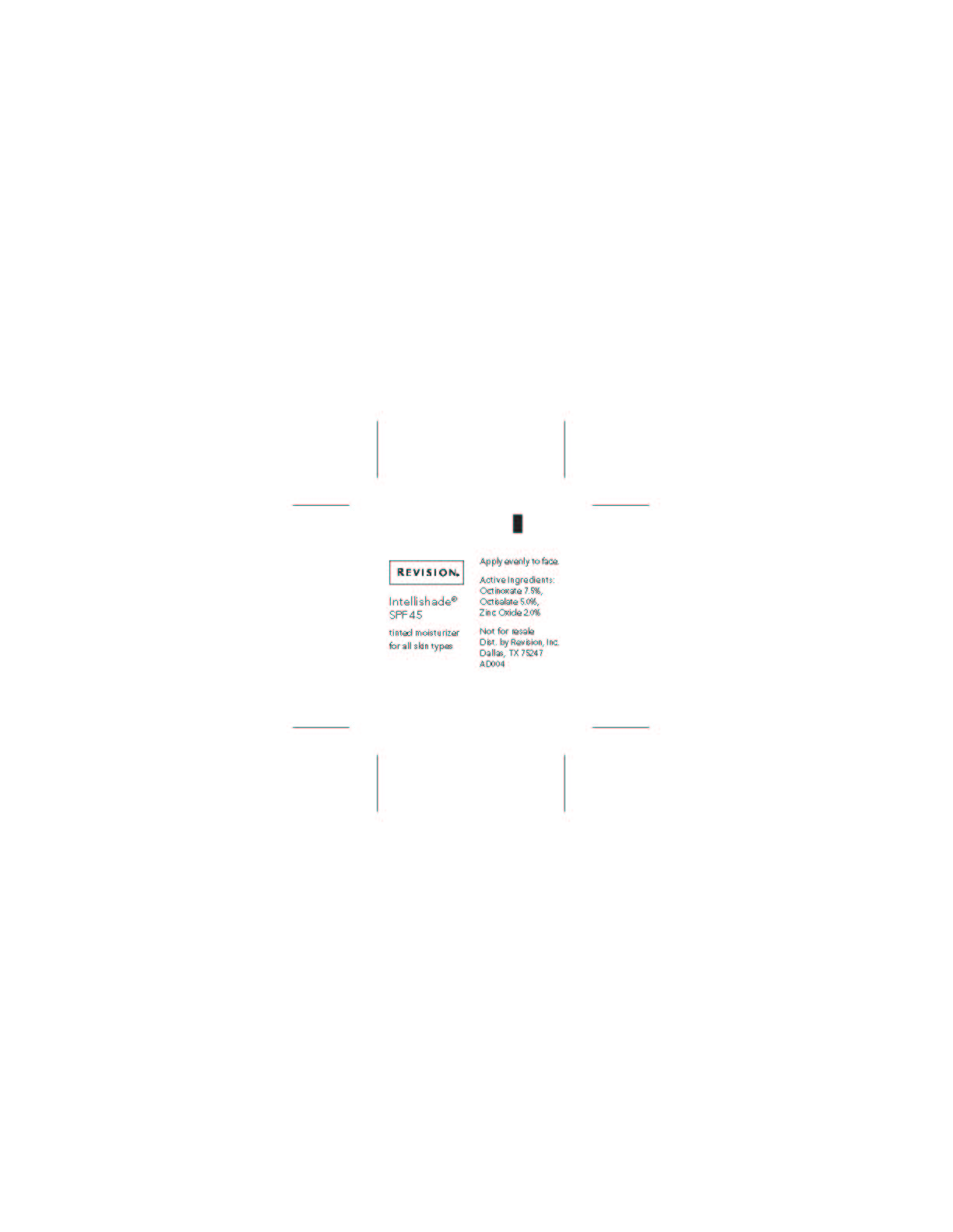
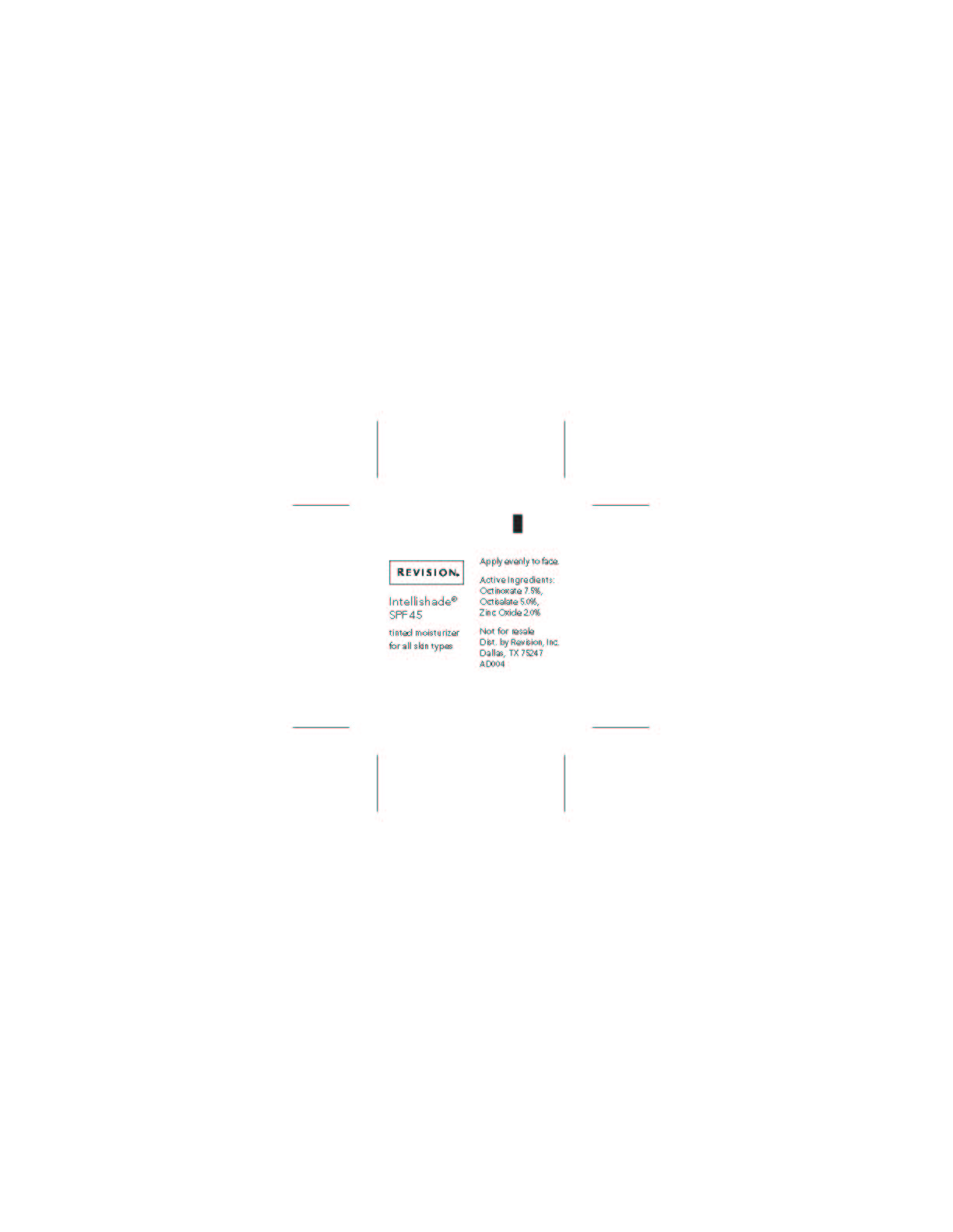
Revercel 1.7oz Retail Triple Duty Sunscreen
triple duty
sunscreen
spf 45
sheer tint
net. wt. 1.7oz / 48g
Active Ingredient Purpose
Octinoxate 7.50% Sunscreen
Octisalate 5.00% Sunscreen
Zinc Oxide 2.00% SunscreenUses
- Helps prevent sunburn.
- Higher SPF gives more sun protection.
- For skin highly sensitive to sunburn.
Keep out of reach of children.
If swallowed, get medical help or contact a
Poison Control Center right away.Directions
- Apply evenly before sun exposure and as needed.
- Children under 6 months of age: ask a doctor.
- Reapply as needed or after towel drying, swimming or perspiring.
Inactive ingredients
Water (Aqua), Cyclopentasiloxane, Ethylhexyl Stearate, Sorbitol,
Caprylic/Capric Triglyceride, Glycerin, Polyglyceryl-4 Isostearate,
Cetyl PEG/PPG 10/1 Dimethicone, Hexyl Laurate, Aloe Barbadensis
Leaf Juice, Cetyl Dimethicone, Betula Alba (White Birch) Extract,
Camellia Sinensis (Green Tea) Leaf Extract, Plankton Extract,
Beeswax, Dimethicone, Squalane, Tocopheryl Acetate, Tetrahexyldecyl
Ascorbate, Hydrogenated Castor Oil, Lecithin, Butylene Glycol, Caprylyl
Glycol, Palmitoyl Tripeptide-5, Saccharomyces Cerevisiae Extract,
Ubiquinone, Retinyl Palmitate, Sodium Chloride, Phenoxyethanol,
Chlorphenesin, Triethoxycaprylylsilane, Benzoic Acid, Sorbic Acid,
Titanium Dioxide, Iron Oxides -
Revision Skincare 8oz Professional Intellishade®
Intellishade®
SPF 45 Original
tinted moisturizer
for all skin types
NT WT 8 OZ / 227 GSkin Type:
Suitable for all skin types to even out skin tone
and protect against sunburn after procedures
such as laser therapy, microdermabrasion and
chemical peels.
Key Ingredients and Benefits:
- Broad spectrum sunscreens protect against UVA and UVB damage
- Coenzyme Q10 and standardized Green Tea Extract provide antioxidant benefits
- Natural minerals even out skin tone and conceal redness
- THD Ascorbate (Vitamin C) and Palmitoyl Tripeptide-5 help reduce the appearance of fine lines and wrinkles
Directions:
Apply liberally to face and neck to help protect skin and conceal redness.
Professional size only. Not for resale.Dist. by Revision, Inc
Dallas, TX 75247 Made in U.S.A.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TINTED SPF 45
octinoxate, octisalate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10477-2627 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) SORBITOL (UNII: 506T60A25R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) HEXYL LAURATE (UNII: 4CG9F9W01Q) ALOE VERA LEAF (UNII: ZY81Z83H0X) BETULA PUBESCENS BARK (UNII: 3R504894L9) GREEN TEA LEAF (UNII: W2ZU1RY8B0) WHITE WAX (UNII: 7G1J5DA97F) DIMETHICONE (UNII: 92RU3N3Y1O) SQUALANE (UNII: GW89575KF9) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PALMITOYL TRIPEPTIDE-5 (UNII: 2A3916MQHO) UBIDECARENONE (UNII: EJ27X76M46) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENZOIC ACID (UNII: 8SKN0B0MIM) SORBIC ACID (UNII: X045WJ989B) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10477-2627-1 1 in 1 CARTON 1 48 g in 1 TUBE 2 NDC:10477-2627-2 227 g in 1 TUBE 3 NDC:10477-2627-3 3 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/01/2007 Labeler - Goodier Cosmetics, LP (007317209) Registrant - Goodier Cosmetics, LP (007317209) Establishment Name Address ID/FEI Business Operations Goodier Cosmetics, LP 007317209 manufacture