Label: ELITE OB PRENATAL MULTIVITAMIN- beta carotene, thiamine mononitrate, riboflavin, pyridoxine hcl, cyanocobalamin, folic acid, cholecalciferol, ascorbic acid, d-alpha succinate, niacinamide, pantothenic acid,gluconate, magnesium oxide, zinc oxide and ferronyl, micronised tablet
- NHRIC Code(s): 13811-027-10
- Packager: Trigen Laboratories, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated December 30, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SUPPLEMENT FACTS
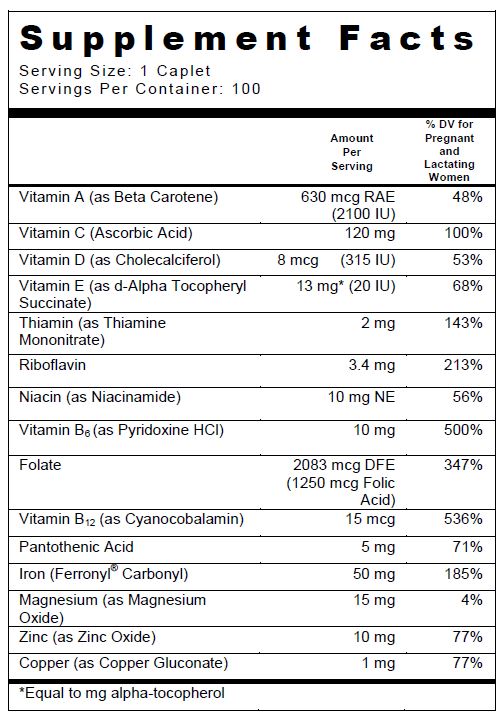
Other Ingredients: Microcrystalline Cellulose, Di Cal Phosphate, Croscarmellose Sodium, Pink Color Coating (Hydroxypropylmethyl Cellulose, Polyvinyl Alcohol, Titanium Dioxide, Polyethylene Glycol, Talc, FD&C Red #40 Lake), Fumed Silica, Stearic Acid, Magnesium Stearate, Povidone K30, Acacia.
GLUTEN, SUGAR, AND LACTOSE FREE
Elite OB™ is a prescription multivitamin/multimineral indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and non-lactating mothers.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Folic acid alone is an improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
- DRUG INTERACTIONS
-
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained herein. However, allergic and idiosyncratic reactions are possible at lower levels. Iron, even at the usual recommended levels, has been associated with gastrointestinal intolerance in some patients.
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
-
PRECAUTIONS
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PLR-EOB-00002-1 Rev. 12/2021
Manufactured for:
Trigen Laboratories, LLC
Alpharetta, GA 30005
www.trigenlab.com

- PRINCIPAL DISPLAY PANEL - 100 tablet label
-
INGREDIENTS AND APPEARANCE
ELITE OB PRENATAL MULTIVITAMIN
beta carotene, thiamine mononitrate, riboflavin, pyridoxine hcl, cyanocobalamin, folic acid, cholecalciferol, ascorbic acid, d-alpha succinate, niacinamide, pantothenic acid,gluconate, magnesium oxide, zinc oxide and ferronyl, micronised tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-027 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 2100 [iU] THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 2 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3.40 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 10 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 15 ug FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1.25 mg VITAMIN D (UNII: 9VU1KI44GP) (CHOLECALCIFEROL - UNII:1C6V77QF41) VITAMIN D 315 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL SUCCINATE, D- 20 [iU] NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 10 mg PANTOTHENIC ACID (UNII: 19F5HK2737) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 5 mg COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 1 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 15 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 10 mg Iron (UNII: E1UOL152H7) (Iron - UNII:E1UOL152H7) Iron 50 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) ACACIA (UNII: 5C5403N26O) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) TALC (UNII: 7SEV7J4R1U) MINERAL OIL (UNII: T5L8T28FGP) FD&C RED NO. 40 (UNII: WZB9127XOA) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-027-10 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 07/13/2013 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color imprint scoring 1 shape size (solid drugs) 19 mm Labeler - Trigen Laboratories, LLC (830479668)


