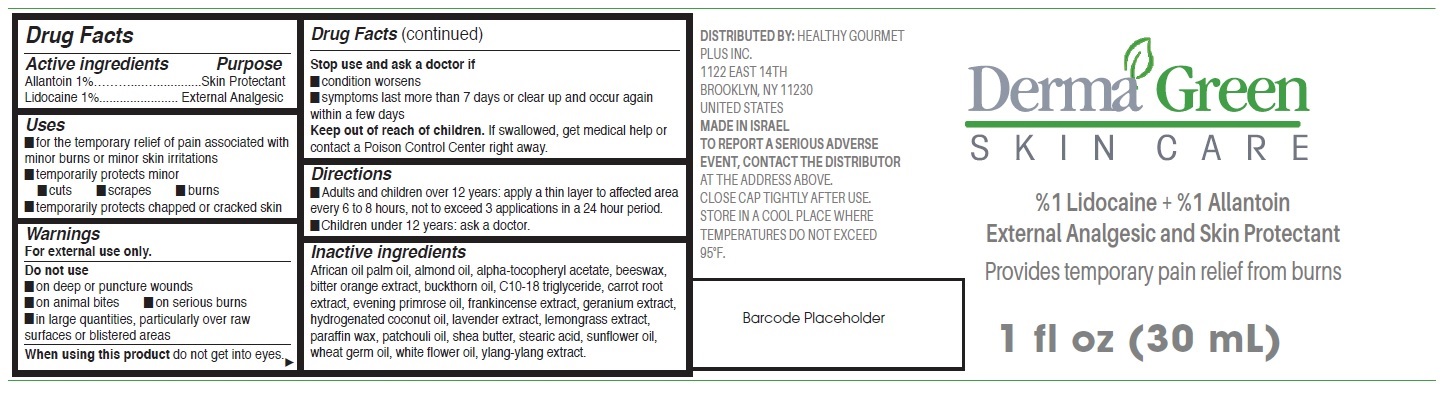
Label: DERMAGREEN SKIN CARE- allantoin, lidocaine cream
- NDC Code(s): 83363-003-00
- Packager: Healthy Gourmet Plus, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
- Directions
-
Inactive ingredients
African oil palm oil, almond oil, alpha-tocopheryl acetate, beeswax, bitter orange extract, buckthorn oil, C10-18 triglyceride, carrot root extract, evening primrose oil, frankincense extract, geranium extract, hydrogenated coconut oil, lavender extract, lemongrass extract, paraffin wax, patchouli oil, shea butter, stearic acid, sunflower oil, wheat germ oil, white flower oil, ylang-ylang extract.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
DERMAGREEN SKIN CARE
allantoin, lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83363-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 10 mg in 1 mL LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALMOND OIL (UNII: 66YXD4DKO9) YELLOW WAX (UNII: 2ZA36H0S2V) CITRUS AURANTIUM FRUIT RIND (UNII: 055456JHI7) C10-18 TRIGLYCERIDES (UNII: 43AGM4PHPI) CARROT (UNII: L56Z1JK48B) EVENING PRIMROSE OIL (UNII: 3Q9L08K71N) FRANKINCENSE (UNII: R9XLF1R1WM) GERANIUM ROBERTIANUM LEAF (UNII: 6H429I0Y3L) HYDROGENATED COCONUT OIL (UNII: JY81OXM1OM) LAVANDULA ANGUSTIFOLIA SUBSP. ANGUSTIFOLIA FLOWER (UNII: 19AH1RAF4M) PARAFFIN (UNII: I9O0E3H2ZE) POGOSTEMON CABLIN LEAF OIL (UNII: F3IN55X5PO) SHEA BUTTER (UNII: K49155WL9Y) STEARIC ACID (UNII: 4ELV7Z65AP) SUNFLOWER OIL (UNII: 3W1JG795YI) WHEAT GERM OIL (UNII: 14C97E680P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83363-003-00 30 mL in 1 JAR; Type 0: Not a Combination Product 11/21/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/21/2023 Labeler - Healthy Gourmet Plus, Inc (083200862)