Label: WATER-BASED PERSONAL LUBRICANT, ACVIOO 001- personal lubricant oil
-
Contains inactivated NDC Code(s)
NDC Code(s): 71847-2215-7 - Packager: Shenzhen Dikailong Technology Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 7, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Inactive ingredient
- Purpose
- When using
- Do not use
- Stop use
- Ask doctor
- Ask doctor/pharmacist
- Keep out of reach of children
- Questions
- Pregnancy or breast feeding
-
Indications & usage
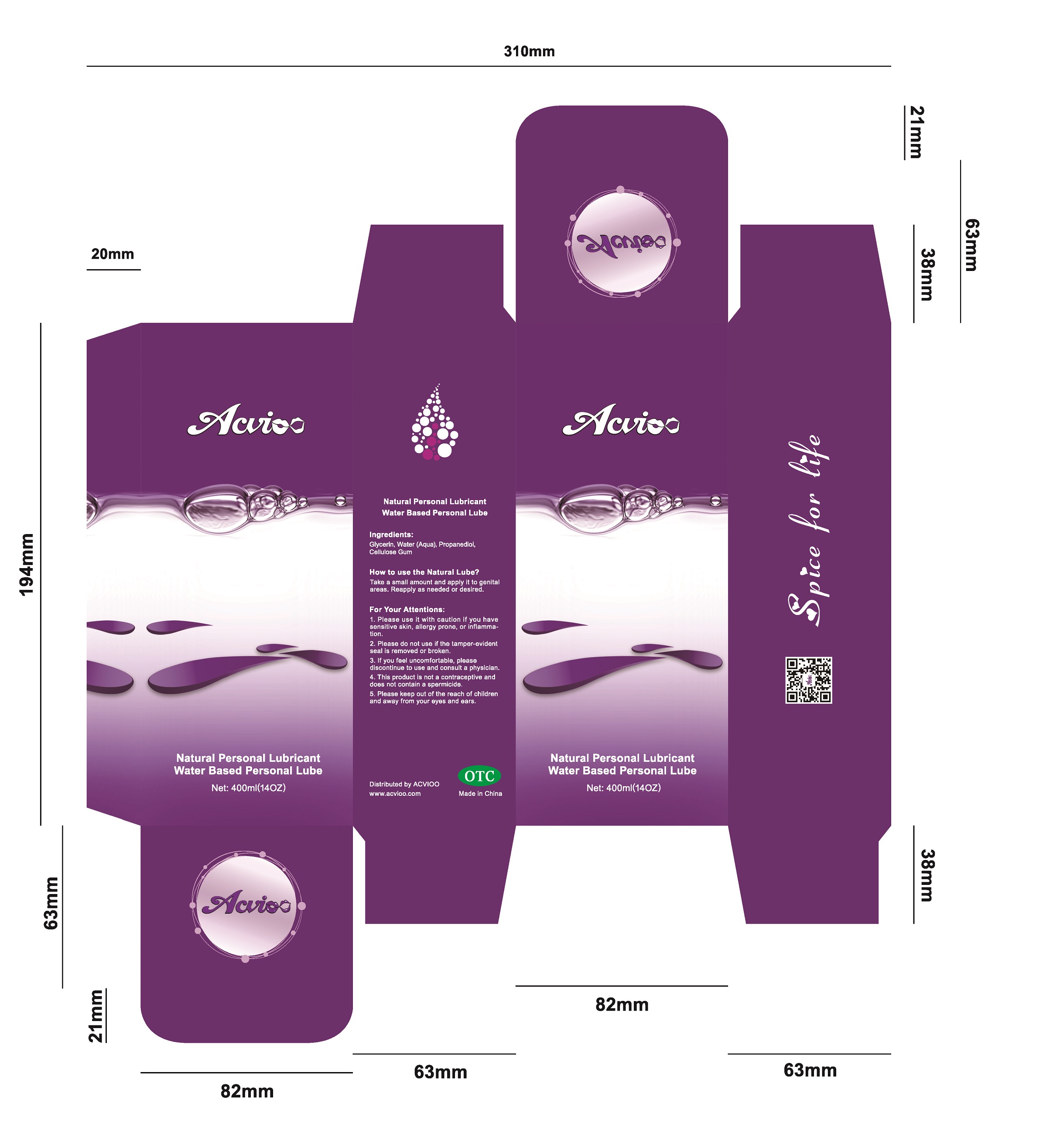
ACVIOO 001 is a water-based personal lubricant, for penile and/or vaginal application, intended to lubricate and moisturize, to enhance the ease and comfort of intimate sexual activity, help relieve virginal dryness, and supplement to the body's natural lubricant.
Apply a small amount to genital areas. Reapply as needed or desired.
- Dosage & administration
- Dosage forms & strengths
- Warnings
- Package label. Principal display panel
-
INGREDIENTS AND APPEARANCE
WATER-BASED PERSONAL LUBRICANT, ACVIOO 001
personal lubricant oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71847-2215 Route of Administration CUTANEOUS, EXTRACORPOREAL, VAGINAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) (BUTYLENE GLYCOL - UNII:3XUS85K0RA) BUTYLENE GLYCOL 80 g in 400 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) 6.4 mL in 400 mL WATER (UNII: 059QF0KO0R) 310.08 mL in 400 mL PHENOXYETHANOL (UNII: HIE492ZZ3T) 1.2 mL in 400 mL POLYETHYLENE GLYCOL 300000 (UNII: 4QIB4U4CQR) 2 mL in 400 mL CHLORPHENESIN (UNII: I670DAL4SZ) 0.32 mL in 400 mL Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71847-2215-7 400 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/07/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/07/2018 Labeler - Shenzhen Dikailong Technology Ltd (544556869) Registrant - Shenzhen Dikailong Technology Ltd (544556869) Establishment Name Address ID/FEI Business Operations Shenzhen Dikailong Technology Ltd 544556869 label(71847-2215) , manufacture(71847-2215)