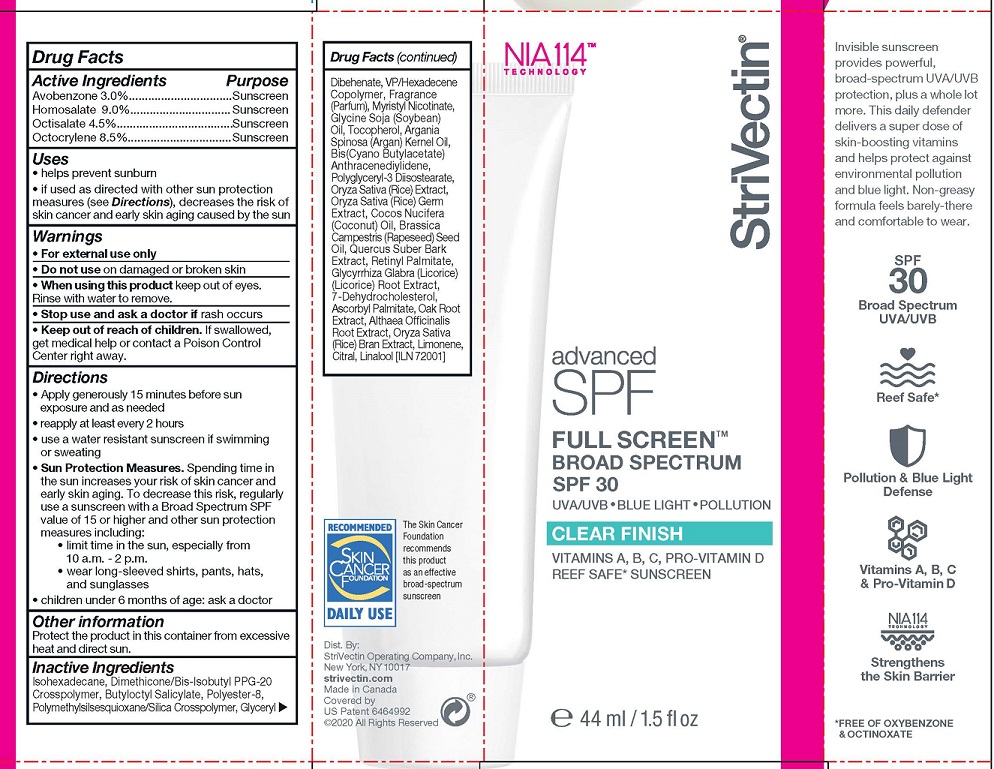
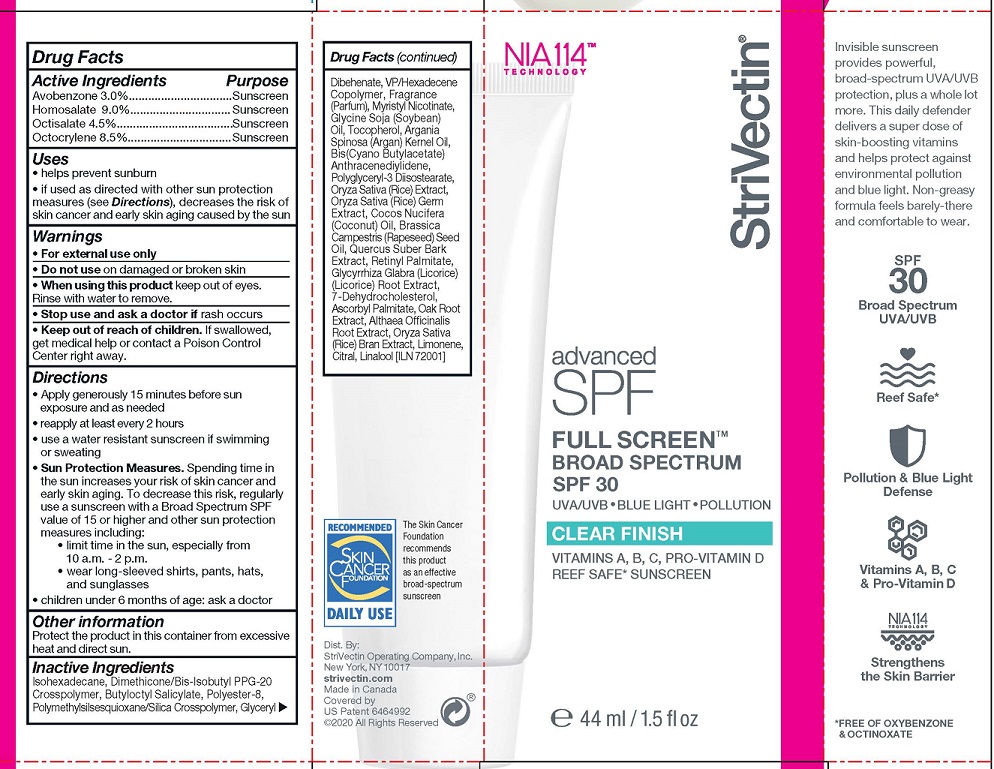
Label: STRIVECTIN ADVANCED SPF FULL SCREEN CLEAR FINISH- avobenzone, homosalate, octisalate, octocrylene lotion
- NDC Code(s): 76147-234-11, 76147-234-13, 76147-234-14
- Packager: StriVectin Operating Company, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- Apply generously 15 minutes before sun exposure and as needed
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time inthe sun increases your risk of skin cancer andearly skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPFvalue of 15 or higher and other sun protectionmeasures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats,and sunglasses
- children under 6 months of age: ask a doctor
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
Isohexadecane, Dimethicone/Bis-Isobutyl PPG-20 Crosspolymer, Butyloctyl Salicylate, Polyester-8, Polymethylsilsesquioxane/Silica Crosspolymer, Glyceryl Dibehenate, VP/Hexadecene Copolymer, Fragrance (Parfum), Myristyl Nicotinate, Glycine Soja (Soybean) Oil, Tocopherol, Argania Spinosa (Argan) Kernel Oil, Bis(Cyano Butylacetate) Anthracenediylidene, Polyglyceryl-3 Diisostearate, Oryza Sativa (Rice) Extract, Oryza Sativa (Rice) Germ Extract, Cocos Nucifera (Coconut) Oil, Brassica Campestris (Rapeseed) Seed Oil, Quercus Suber Bark Extract, Retinyl Palmitate, Glycyrrhiza Glabra (Licorice) (Licorice) Root Extract, 7-Dehydrocholesterol, Ascorbyl Palmitate, Oak Root Extract, Althaea Officinalis Root Extract, Oryza Sativa (Rice) Bran Extract, Limonene, Citral, Linalool
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STRIVECTIN ADVANCED SPF FULL SCREEN CLEAR FINISH
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76147-234 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 8.5 g in 100 mL Inactive Ingredients Ingredient Name Strength ISOHEXADECANE (UNII: 918X1OUF1E) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) MYRISTYL NICOTINATE (UNII: 8QWM6I035C) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) ARGAN OIL (UNII: 4V59G5UW9X) BUTYL ACETATE (UNII: 464P5N1905) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) ORYZA SATIVA WHOLE (UNII: 84IVV0906Z) COCONUT OIL (UNII: Q9L0O73W7L) CANOLA OIL (UNII: 331KBJ17RK) QUERCUS SUBER BARK (UNII: 8R5219271Q) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) LICORICE (UNII: 61ZBX54883) 7-DEHYDROCHOLESTEROL (UNII: BK1IU07GKF) ASCORBYL PALMITATE (UNII: QN83US2B0N) QUERCUS SPP. WHOLE (UNII: MF4E5I2OUQ) ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) LIMONENE, (+)- (UNII: GFD7C86Q1W) CITRAL (UNII: T7EU0O9VPP) LINALOOL, (+)- (UNII: F4VNO44C09) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76147-234-11 1 in 1 BOX 12/04/2019 1 44 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:76147-234-13 1 mL in 1 PACKET; Type 0: Not a Combination Product 12/04/2019 3 NDC:76147-234-14 7 mL in 1 TUBE; Type 0: Not a Combination Product 12/13/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/04/2019 Labeler - StriVectin Operating Company, Inc. (832343722) Registrant - StriVectin Operating Company, Inc. (832343722)