Label: CLEASE AND TREAT- benzoyl peroxide pad and salicylic acid pad cloth
-
Contains inactivated NDC Code(s)
NDC Code(s): 23710-052-02 - Packager: Quinnova Pharmaceuticals, Inc.
- Category: NON-STANDARDIZED ALLERGENIC LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
Drug Label Information
Updated December 11, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Cleanse & Treat
(benzoyl peroxide pad and salicylic acid pad)
Rx OnlyCleanse & Treat contains two separate and distinct non-woven pads enclosed in a heat-sealed foil pack compartmentalized from one another within the foil pack by filmstock. One non-woven pad bears a preparation consisting of 5% benzoyl peroxide and the following inactive ingredients: ammonium lauryl sulfate, carbomer, disodium cocoamphodiacetate, DMDM hydantoin, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium chloride, sodium hydroxide. The other non-woven pad bears a preparation consisting of 2% salicylic acid and the following inactive ingredients: ammonium lauryl sulfate, cellulose gum, disodium ricinoleamido MEA-sulfosuccinate, DMDM hydantoin, propylene glycol monoricinoleate, purified water, sodium citrate dihydrate.
CHEMICAL STRUCTURE
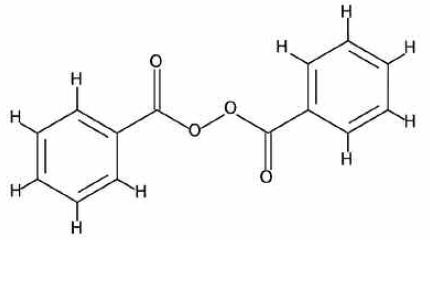
Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. It has an empirical formula of C14H10O4 with a molecular weight of 242.23. Benzoyl peroxide has the following molecular structure:
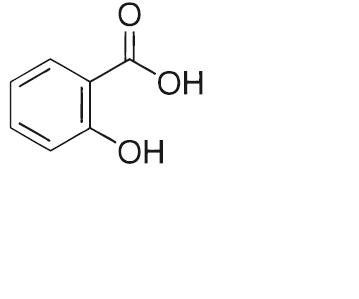
Salicylic acid is the 2 hydroxy derivative of benzoic aid. It has an empirical formula of C7H6O3 with a molecular weight of 138.12. Salicylic acid has the following molecular structure:
-
CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris is not known, however, its antibacterial activity against Propionibacterium acnes, together with its mild keratolytic effect, is believed to be its significant mode of action.
Information for Patients - Patients should avoid unnecessary sun exposure and use a sunscreen when in sunlight. Contact with hair, fabrics, or any colored materials may result in bleaching or discoloration. Patients should avoid concomitant use of other drugs that may contribute to elevated serum salicylate levels where the potential for toxicity exists. Symptoms of salicylate toxicity may include nausea, vomiting, dizziness, loss of hearing, tinnitus, lethargy, hyperpnea and diarrhea. In the event of salicylate toxicity, the use of Cleanse & Treat should be discontinued, fluids should be administered to promote urinary excretion and medical assistance should be obtained immediately.
Carcinogenesis, Mutagenesis, Impairment of Fertility - Data from some studies using a strain of mice highly susceptible to eveloping cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of these findings to humans is not known. Benzoyl peroxide has not been found to be mutagenic in the Ames Salmonella Test and there are no published data suggesting that it impairs fertility. No data are available concerning potential carcinogenic or reproductive effects of salicylic acid. It has not been found to be mutagenic in the Ames Salmonella Test and there are no published data suggesting that it impairs fertility.
Pregnancy (Category C) - Animal reproduction studies have not been performed with benzoyl peroxide and it is not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman. Nevertheless, benzoyl peroxide should be used by a pregnant woman only if necessary. Salicylic acid has been shown to be teratogenic in rats and monkeys. It is difficult to extrapolate from oral doses of acetylsalicylic acid used in these studies to topical administration as the oral dose to monkeys may represent 10 times or more the maximum daily human dose of salicylic acid when applied topically as directed with Cleanse &Treat. There are no adequate and well-controlled studies in pregnant women. Nevertheless, salicylic acid should be used by a pregnant woman only if necessary.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- ADVERSE REACTIONS
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Unless otherwise directed by a prescribing physician, patients should apply one salicylic acid pad and one benzoyl peroxide pad to affected areas twice per day. Cleanse & Treat is a leave-on acne treatment, intended for use without water or additional cleansers unless otherwise directed by your physician.
-
HOW SUPPLIED
Each Cleanse & Treat packette consists of one 0.8 g. 5% benzoyl peroxide pad and one 0.5 g. 2% salicylic acid pad, with each pad separated from one another by filmstock and both pads enclosed together in an individual heat-sealed foil pack bearing the NDC Number 23710-052-02.
Store at 15°-25° C (59°-77° F)
- REFERENCES
- Pouch
-
INGREDIENTS AND APPEARANCE
CLEASE AND TREAT
benzoyl peroxide pad and salicylic acid pad clothProduct Information Product Type NON-STANDARDIZED ALLERGENIC Item Code (Source) NDC:23710-052 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 0.8 g in 5 g SELENIUM SULFIDE (UNII: Z69D9E381Q) (SELENIUM - UNII:H6241UJ22B) SELENIUM SULFIDE 0.5 g in 5 g Inactive Ingredients Ingredient Name Strength AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) CARBOMER 934 (UNII: Z135WT9208) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) POWDERED CELLULOSE (UNII: SMD1X3XO9M) GLYCERYL RICINOLEATE (UNII: ZUE0CEL42O) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23710-052-02 5 g in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 01/01/2009 Labeler - Quinnova Pharmaceuticals, Inc. (607183766)

 CLNSTR011 4/08
CLNSTR011 4/08