Label: ECOLAB- alcohol solution
- NDC Code(s): 47593-472-41, 47593-472-55, 47593-472-56
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- INACTIVE INGREDIENT
- QUESTIONS
-
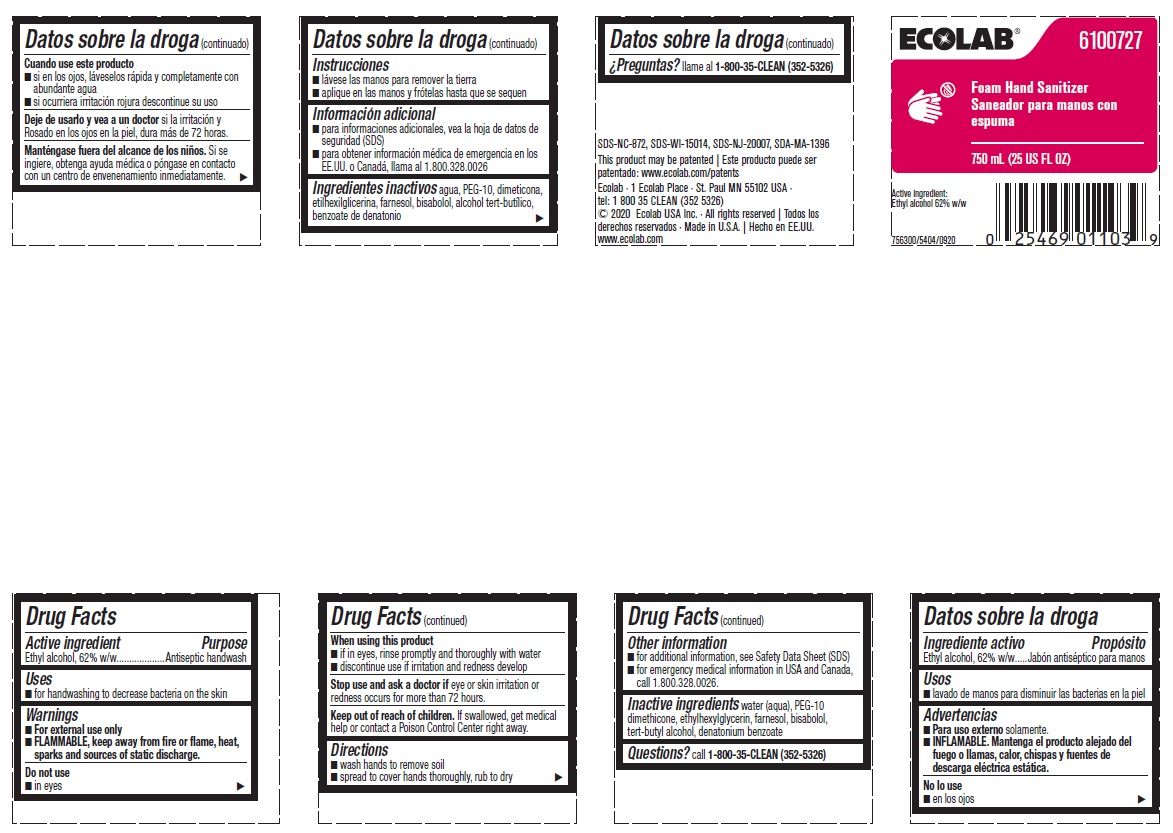
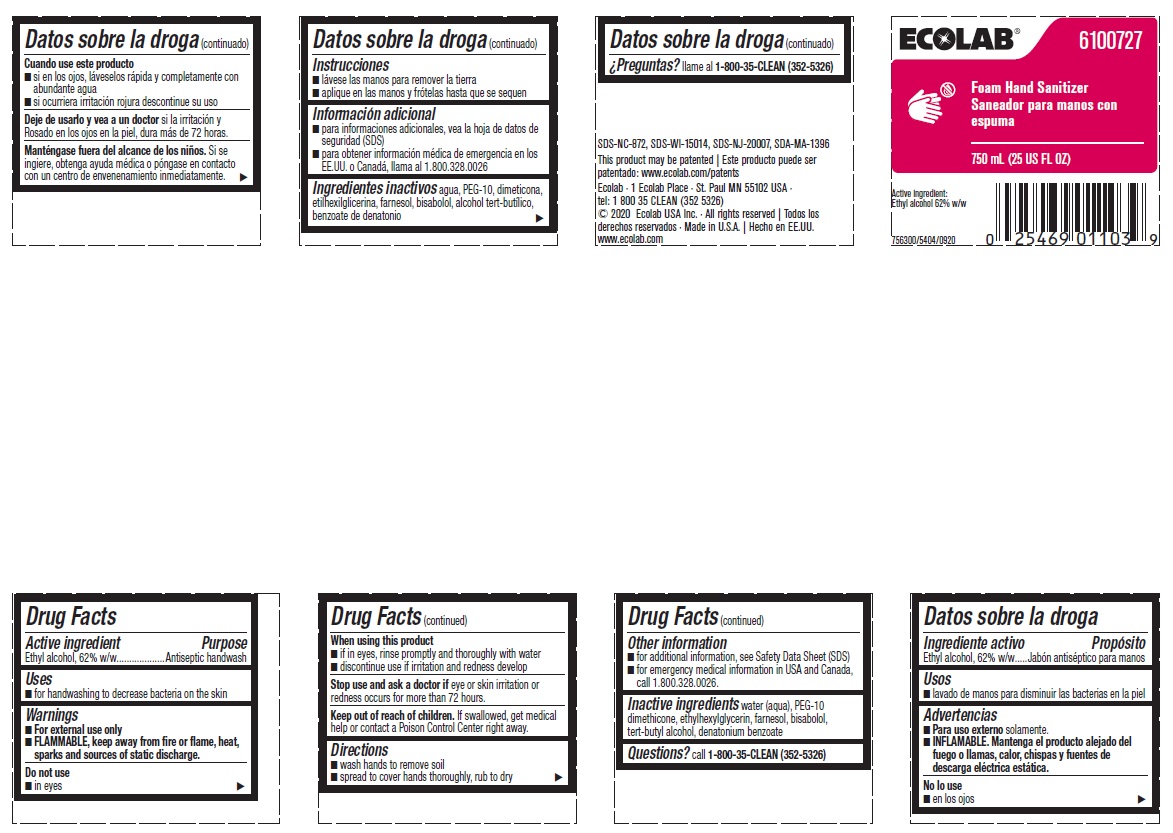
Principal display panel and representative label
ECOLAB® 6100727
Foam Hand Sanitizer
750 ML (25 US FL OZ)
Active Ingredient:
Ethyl aclohol, 62% w/w
756300/5404/0920
SDS-NC-872, SDS-WI-15014, SDS-NJ-20007, SDA-MA-1396
This product may be patented | Este producto puede ser
patentado: www.ecolab.com/patents
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA ·
tel: 1 800 35 CLEAN (352 5326)
© 2020 Ecolab USA Inc. · All rights reserved | Todos los derechos reservados · Made in U.S.A. | Hecho en EE.UU. www.ecolab.com
-
INGREDIENTS AND APPEARANCE
ECOLAB
alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-472 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) FARNESOL (UNII: EB41QIU6JL) LEVOMENOL (UNII: 24WE03BX2T) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-472-55 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2010 09/28/2021 2 NDC:47593-472-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/24/2013 3 NDC:47593-472-56 1200 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/24/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 11/16/2010 Labeler - Ecolab Inc. (006154611)