Label: chloromycetin- chloramphenicol ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 0071-3070-07 - Packager: PARKE-DAVIS
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 8, 2006
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
- BOXED WARNING (What is this?)
-
Description
Each gram of Chloromycetin Ophthalmic Ointment, 1% contains 10 mg of chloramphenicol in a special base of liquid petrolatum and polyethylene. It contains no preservatives. Sterile ointment.
The chemical names for chloramphenicol are:
(1) Acetamide, 2,2-dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl) ethyl]-, and
(2) D-threo-(—)-2,2-Dichloro-N-[β-hydroxy-α-(hydroxymethyl)-p-nitrophenethyl] acetamide.
Chloramphenicol has the following empirical and structural formulas:
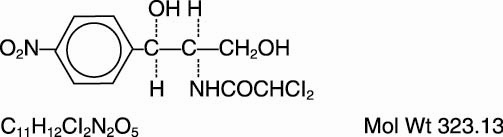
-
Clinical Pharmacology
Chloramphenicol is a broad-spectrum antibiotic originally isolated from Streptomyces venezuelae. It is primarily bacteriostatic and acts by inhibition of protein synthesis by interfering with the transfer of activated amino acids from soluble RNA to ribosomes. It has been noted that chloramphenicol is found in measurable amounts in the aqueous humor following local application to the eye. Development of resistance to chloramphenicol can be regarded as minimal for staphylococci and many other species of bacteria.
-
Indications and Usage
Chloramphenicol should be used only in those serious infections for which less potentially dangerous drugs are ineffective or contraindicated. Bacteriological studies should be performed to determine the causative organisms and their sensitivity to chloramphenicol (see Boxed Warning).
Chloromycetin Ophthalmic Ointment, 1% (Chloramphenicol Ophthalmic Ointment, USP) is indicated for the treatment of surface ocular infections involving the conjunctiva and/or cornea caused by chloramphenicol-susceptible organisms.
The particular antiinfective drug in this product is active against the following common bacterial eye pathogens:
Staphylococcus aureus
Streptococcus, including Streptococcus pneumoniae
Escherichia coli
Haemophilus influenzae
Klebsiella/Enterobacter species
Moraxella lucunata
(Morax-Axenfeld bacillus)
Neisseria species
This product does not provide adequate coverage against:
Pseudomonas aeruginosa
Serratia marcescens
- Contraindications
- Warnings
-
Precautions
The prolonged use of antibiotics may occasionally result in overgrowth of nonsusceptible organisms including fungi. If new infections appear during medication, the drug should be discontinued and appropriate measures should be taken.
In all serious infections the topical use of chloramphenicol should be supplemented by appropriate systemic medication.
-
Adverse Reactions
Blood dyscrasias have been reported in association with the use of chloramphenicol (see WARNINGS).
Allergic or inflammatory reactions due to individual hypersensitivity and occasional burning or stinging may occur with the use of Chloromycetin Ophthalmic Ointment.
-
Dosage and Administration
A small amount of ointment placed in the lower conjunctival sac every three hours, or more frequently if deemed advisable by the prescribing physician. Administration should be continued day and night the first 48 hours, after which the interval between applications may be increased. Treatment should be continued for at least 48 hours after the eye appears normal.
-
How Supplied
N 0071–3070–07
Chloromycetin Ophthalmic Ointment, 1% (Chloramphenicol Ophthalmic Ointment, USP) is supplied, sterile, in ophthalmic ointment tubes of 3.5 grams.
Chloromycetin, brand of chloramphenicol. Reg US Pat Off
Caution—Federal law prohibits dispensing without prescription.March 1997
©1997, Warner-Lambert Co.
PARKE-DAVIS
Div of Warner-Lambert Co/Morris Plains, NJ 07950 USA
-
INGREDIENTS AND APPEARANCE
CHLOROMYCETIN
chloramphenicol ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0071-3070 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength chloramphenicol (UNII: 66974FR9Q1) (chloramphenicol - UNII:66974FR9Q1) 10 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0071-3070-07 3.5 g in 1 TUBE Labeler - PARKE-DAVIS
