Label: OPSYNVI- macitentan and tadalafil tablet, film coated
-
NDC Code(s):
66215-812-01,
66215-812-07,
66215-812-08,
66215-812-30, view more66215-814-01, 66215-814-10, 66215-814-30
- Packager: Actelion Pharmaceuticals US, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OPSYNVI safely and effectively. See full prescribing information for OPSYNVI.
OPSYNVI ®(macitentan and tadalafil) tablets, for oral use
Initial U.S. Approval: 2024WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete boxed warning.
- Based on animal data, OPSYNVI may cause fetal harm if used during pregnancy ( 4.1, 5.1, 8.1).
- Females of reproductive potential: exclude pregnancy before start of treatment. Prevent pregnancy prior to initiation of treatment, during treatment and for one month after treatment by using effective methods of contraception ( 2.2, 8.3).
- When pregnancy is detected, discontinue OPSYNVI as soon as possible ( 5.1).
RECENT MAJOR CHANGES
Boxed Warning 4/2025 Dosage and Administration ( 2.2) 4/2025 Warnings and Precautions ( 5.1) 4/2025 Warnings and Precautions (5.2) Removal 4/2025 INDICATIONS AND USAGE
OPSYNVI is a combination of macitentan, an endothelin receptor antagonist (ERA), and tadalafil, a phosphodiesterase 5 (PDE5) inhibitor, indicated for chronic treatment of pulmonary arterial hypertension (PAH, WHO Group I) in adult patients of WHO functional class (FC) II–III. ( 1.1)
Individually, macitentan reduces the risk of clinical worsening events and hospitalization, and tadalafil improves exercise ability. ( 1.1, 14)
DOSAGE AND ADMINISTRATION
- One 10 mg/20 mg or 10 mg/40 mg tablet taken orally once daily with or without food. ( 2.1)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hepatotoxicity: ERAs cause hepatotoxicity and liver failure. Obtain baseline liver enzymes and monitor as clinically indicated. ( 5.2)
- Hypotension: Vasodilatory effects may cause hypotension in susceptible patients. ( 5.3)
- Hemoglobin decrease. ( 5.4)
- Worsening Pulmonary Veno-Occlusive Disease: If pulmonary edema is confirmed, discontinue treatment. ( 5.5)
- Visual Loss: Sudden loss of vision could be a sign of non-arteritic ischemic optic neuropathy (NAION) and may be permanent. ( 5.6)
- Hearing Impairment: Cases of sudden decrease or loss of hearing have been reported in patients taking tadalafil. ( 5.7)
- Fluid Retention: Fluid retention may require intervention. ( 5.8)
- Combination with Other PDE5 Inhibitors: Avoid use with other PDE5 inhibitors. ( 5.9)
- Decreased Sperm Count: Decreases in sperm count have been observed in patients taking ERAs. ( 5.10)
- Prolonged Erection: Advise patients to seek emergency treatment if an erection lasts greater than 4 hours. ( 5.11)
ADVERSE REACTIONS
Most common adverse reactions (≥10%) are edema/fluid retention, anemia, and headache/migraine. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: EMBRYO-FETAL TOXICITY
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Pregnancy Testing in Females of Reproductive Potential
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Pregnancy
4.2 Hypersensitivity
4.3 Concomitant Organic Nitrates
4.4 Concomitant Guanylate Cyclase (GC) Stimulators
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-fetal Toxicity
5.2 Hepatotoxicity
5.3 Hypotension
5.4 Hemoglobin Decrease
5.5 Worsening Pulmonary Veno-Occlusive Disease (PVOD)
5.6 Visual Loss
5.7 Hearing Impairment
5.8 Fluid Retention
5.9 Combination with Other PDE5 Inhibitors
5.10 Decreased Sperm Count
5.11 Prolonged Erection
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Nitrates
7.2 Strong CYP3A4 Inducers
7.3 Strong CYP3A4 Inhibitors
7.4 Moderate Dual or Combined CYP3A4 and CYP2C9 Inhibitors
7.5 Alpha-Blockers
7.6 Antihypertensives
7.7 Alcohol
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Pulmonary Arterial Hypertension
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY
OPSYNVI is contraindicated for use during pregnancy because it may cause fetal harm based on animal data [see Contraindications (4.1), Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
Therefore, for females of reproductive potential, exclude pregnancy before the start of treatment with OPSYNVI. Advise use of effective contraception before the initiation of treatment, during treatment, and for one month after stopping treatment with OPSYNVI [see Dosage and Administration (2.2), Use in Specific Populations (8.3)]. When pregnancy is detected, discontinue OPSYNVI as soon as possible [see Warnings and Precautions (5.1)] .
-
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
OPSYNVI is the combination of macitentan and tadalafil indicated for the chronic treatment of adults with pulmonary arterial hypertension (PAH, WHO Group I and WHO Functional Class (FC) II–III).
Individually, macitentan reduces the risk of clinical worsening events and hospitalization, and tadalafil improves exercise ability [see Clinical Studies (14.1)] .
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
OPSYNVI is taken orally once daily with or without food. Swallow the tablets whole, with water. Do not cut, crush, or chew tablets. If the patient misses a dose of OPSYNVI, tell the patient to take it as soon as possible and then take the next dose at the regularly scheduled time. Tell the patient not to take two doses at the same time if a dose has been missed.
For patients who are treatment-naïve to any PAH specific therapy or transitioning from ERA monotherapy
The recommended starting dose of OPSYNVI is one 10 mg/20 mg tablet taken orally once daily with or without food for one week. If tolerated, up titrate OPSYNVI to one 10 mg/40 mg tablet taken orally once daily with or without food as the maintenance dose.
2.2 Pregnancy Testing in Females of Reproductive Potential
Exclude pregnancy before initiating treatment with OPSYNVI in females of reproductive potential [see Boxed Warning, Contraindications (4.1), Warnings and Precautions (5.1), and Use in Specific Populations (8.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Pregnancy
OPSYNVI may cause fetal harm when administered to a pregnant woman. OPSYNVI is contraindicated in females who are pregnant. Macitentan was consistently shown to have teratogenic effects when administered to animals. If OPSYNVI is used during pregnancy, advise the patient of the potential risk to a fetus [see Warnings and Precautions (5.1)and Use in Specific Populations (8.1)] .
4.2 Hypersensitivity
OPSYNVI is contraindicated in patients with a history of a hypersensitivity reaction to macitentan, tadalafil, or any component of the product. Hypersensitivity reactions have been reported. Stevens-Johnson syndrome and exfoliative dermatitis have been reported with tadalafil [see Adverse Reactions (6.2)].
4.3 Concomitant Organic Nitrates
OPSYNVI is contraindicated in patients who are using any form of organic nitrate, either regularly or intermittently. Do not use nitrates within 48 hours of the last dose of OPSYNVI. Tadalafil potentiates the hypotensive effect of nitrates. This potentiation is thought to result from the combined effects of nitrates and tadalafil on the nitric oxide/cGMP pathway [see Clinical Pharmacology (12.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-fetal Toxicity
Based on data from animal reproduction studies, OPSYNVI may cause fetal harm when administered to a pregnant patient and is contraindicated during pregnancy. The available human data for ERAs do not establish the presence or absence of major birth defects related to the use of OPSYNVI. Advise patients who can become pregnant of the potential risk to a fetus. Obtain a pregnancy test prior to initiation of therapy with OPSYNVI. Advise patients who can become pregnant to use effective contraceptive methods prior to initiation of treatment, during treatment, and for one month after discontinuation of treatment with OPSYNVI. When pregnancy is detected, discontinue use as soon as possible [see Dosage and Administration (2.2), Contraindications (4.1), and Use in Specific Populations (8.1, 8.3)].
5.2 Hepatotoxicity
ERAs have caused elevations of aminotransferases, hepatotoxicity, and liver failure.
The incidence of elevated aminotransferases in the double-blind and combined double-blind (DB)/open-label (OL) arms of the study of OPSYNVI in PAH are shown in Table 1.
Table 1: Incidence of Elevated Aminotransferases in the A DUE Study OPSYNVI DB
(N=107)OPSYNVI DB/OL
(N=185)≥3 × ULN 1.0% 3.4% ≥8 × ULN 1.0% 1.1% The overall incidence of treatment discontinuations for hepatic adverse events in the double-blind and combined double-blind/open-label arms study of OPSYNVI in PAH data were 0.9% and 2.2% respectively.
The incidence of elevated aminotransferases in the study of OPSUMIT (macitentan) in PAH is shown in Table 2.
Table 2: Incidence of Elevated Aminotransferases in the SERAPHIN Study OPSUMIT 10 mg
(N=242)Placebo
(N=249)>3 × ULN 3.4% 4.5% >8 × ULN 2.1% 0.4% In the placebo-controlled study of OPSUMIT, discontinuations for hepatic adverse events were 3.3% in the OPSUMIT 10 mg group vs. 1.6% for placebo.
Obtain liver enzyme tests prior to initiation of OPSYNVI and repeat during treatment as clinically indicated.
Advise patients to report symptoms suggesting hepatic injury (nausea, vomiting, right upper quadrant pain, fatigue, anorexia, jaundice, dark urine, fever, or itching). If clinically relevant aminotransferase elevations occur, or if elevations are accompanied by an increase in bilirubin >2 × ULN, or by clinical symptoms of hepatotoxicity, discontinue OPSYNVI. Consider re-initiation of OPSYNVI when hepatic enzyme levels normalize in patients who have not experienced clinical symptoms of hepatotoxicity.
Do not initiate OPSYNVI in patients with elevated aminotransferases (> 3 × upper limit of normal [ULN]) at baseline. Patients with severe hepatic cirrhosis (Child-Pugh Class C) have not been studied, and, therefore, avoid use of OPSYNVI.
5.3 Hypotension
OPSYNVI tablets have vasodilatory properties that may result in transient decreases in blood pressure. Prior to prescribing OPSYNVI tablets, carefully consider whether patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects. Patients with pre-existing hypotension, with autonomic dysfunction, with left ventricular outflow obstruction, may be particularly sensitive to the actions of vasodilators [see Clinical Pharmacology (12.2)].
5.4 Hemoglobin Decrease
Decreases in hemoglobin concentration and hematocrit have occurred following administration of other ERAs and were observed in clinical studies with OPSYNVI and OPSUMIT. These decreases occurred early and stabilized thereafter.
In the placebo-controlled study of OPSUMIT in PAH, OPSUMIT 10 mg caused a mean decrease in hemoglobin from baseline to up to 18 months of about 1.0 g/dL compared to no change in the placebo group. A decrease in hemoglobin to below 10.0 g/dL was reported in 8.7% of the OPSUMIT 10 mg group and in 3.4% of the placebo group. Similar results were observed in the trial with OPSYNVI.
Decreases in hemoglobin seldom require transfusion. Initiation of OPSYNVI is not recommended in patients with severe anemia. Measure hemoglobin prior to initiation of treatment and repeat during treatment as clinically indicated [see Adverse Reactions (6.1)].
5.5 Worsening Pulmonary Veno-Occlusive Disease (PVOD)
Pulmonary vasodilators may significantly worsen the cardiovascular status of patients with pulmonary veno-occlusive disease (PVOD). Since there are no clinical data on administration of OPSYNVI tablets to patients with veno-occlusive disease, administration of OPSYNVI tablets to such patients is not recommended. Should signs of pulmonary edema occur when OPSYNVI tablets are administered, the possibility of associated PVOD should be considered. If confirmed, discontinue OPSYNVI.
5.6 Visual Loss
Non–arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision, including permanent loss of vision, has been reported postmarketing in temporal association with the use of PDE5 inhibitors, including tadalafil. Most, but not all, of these patients had underlying anatomic or vascular risk factors for development of NAION, including: low cup to disc ratio ("crowded disc"), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia, and smoking. Based on published literature, the annual incidence of NAION is 2.5–11.8 cases per 100,000 in males aged greater than or equal to 50 in the general population. Other risk factors for NAION, such as the presence of "crowded" optic disc, may have contributed to the occurrence of NAION.
Patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa, were not included in the clinical trials, and use of OPSYNVI in these patients is not recommended.
5.7 Hearing Impairment
Cases of sudden decrease or loss of hearing, which may be accompanied by tinnitus and dizziness, have been reported in patients taking tadalafil. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors.
5.8 Fluid Retention
Peripheral edema and fluid retention are known clinical consequences of PAH and known effects of ERAs and heart failure has been reported in patients taking OPSYNVI. In the active-controlled and combined double-blind/open-label arms of the study of OPSYNVI in PAH, the incidence of peripheral edema/fluid retention was 20.6% in the active-controlled and 17.3% in the double-blind/open-label arm [see Adverse Reactions (6.1)] . In the placebo-controlled study of OPSUMIT in PAH, the incidence of edema was 21.9% in the OPSUMIT 10 mg group and 20.5% in the placebo group.
Patients with underlying left ventricular dysfunction may be at particular risk for developing significant fluid retention after initiation of ERA treatment. In a small study of OPSUMIT in patients with pulmonary hypertension because of left ventricular dysfunction, more patients in the OPSUMIT group developed significant fluid retention and had more hospitalizations because of worsening heart failure compared to those randomized to placebo. Postmarketing cases of edema and fluid retention occurring within weeks of starting OPSUMIT, some requiring intervention with a diuretic or hospitalization for decompensated heart failure, have been reported .
Monitor for signs of fluid retention after OPSYNVI initiation. If clinically significant fluid retention develops, evaluate the patient to determine the cause, such as OPSYNVI or underlying heart failure, and the possible need to discontinue OPSYNVI.
5.9 Combination with Other PDE5 Inhibitors
Tadalafil is also indicated for erectile dysfunction. The safety and efficacy of taking tadalafil tablets together with another PDE5 inhibitors or other treatments for erectile dysfunction have not been studied. Instruct patients taking OPSYNVI tablets not to take other PDE5 inhibitors.
5.10 Decreased Sperm Count
Macitentan, like other ERAs, may have an adverse effect on spermatogenesis. Counsel men about potential effects on fertility [see Use in Specific Populations (8.3)and Nonclinical Toxicology (13.1)].
5.11 Prolonged Erection
There have been reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for PDE5 inhibitors like tadalafil. Patients with conditions that might predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia), or in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis, or Peyronie's disease) are at an increased risk. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Patients who have an erection lasting greater than 4 hours, whether painful or not, should seek emergency medical attention.
-
6 ADVERSE REACTIONS
Clinically significant adverse reactions that appear in other sections of the labeling include:
- Hypersensitivity [see Contraindications (4.2)]
- Embryo-fetal Toxicity [see Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Hypotension [see Warnings and Precautions (5.3)]
- Decrease in Hemoglobin [see Warnings and Precautions (5.4)]
- Visual Loss [see Warnings and Precautions (5.6)and Patient Counseling Information (17)]
- Hearing loss [see Warnings and Precautions (5.7)]
- Fluid Retention [see Warnings and Precautions (5.8)]
- Prolonged Erection [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The overall safety profile of OPSYNVI is based on data from a double-blind, active-controlled, phase 3 clinical study (A DUE) and an open-label extension study, in patients with PAH [see Clinical Studies (14)] . In the double-blind portion of the study, a total of 107 patients were treated with OPSYNVI 10 mg/40 mg, 35 patients were treated with 10 mg macitentan monotherapy, and 44 patients were treated with 40 mg tadalafil monotherapy. The duration of exposure to OPSYNVI during the double-blind portion was 16 weeks.
The most common adverse reactions (occurring in ≥ 10% of the OPSYNVI-treated patients) from the double-blind study data were edema/fluid retention (21%), anemia (19%), and headache/migraine (18%). The incidence of treatment discontinuations due to adverse events among patients receiving OPSYNVI in the double-blind phase of the study was 8%. The most frequent adverse reactions leading to discontinuation were anemia and hemoglobin decreased (2% grouped) and peripheral edema and peripheral swelling (2% grouped). Table 3 presents adverse reactions seen in patients treated for 16 weeks during the double-blind portion of A DUE.
Table 3: Adverse Reactions Occurring in 3% or More of Patients Treated with OPSYNVI During the 16-week Double-blind Study Portion of A DUE Adverse Reaction OPSYNVI
N=107
%Macitentan Monotherapy
N=35
%Tadalafil Monotherapy
N=44
%Edema/fluid retention 21 14 16 Anemia 19 3 2 Headache 18 17 14 Abdominal pain 7 3 14 Hypotension 7 0 0 Myalgia 6 0 5 Nasopharyngitis 6 3 0 Nausea 6 0 7 Increased uterine bleeding 5 0 0 Back pain 5 3 9 Flushing 4 6 0 Vomiting 4 0 5 Palpitations 4 3 5 Pain in extremity 3 0 7 Epistaxis 3 0 0 One-hundred eighty-five patients received OPSYNVI in the double-blind or open-label phase of the study. The median exposure to OPSYNVI during the combined double-blind/open-label extension was 59.9 weeks with a mean exposure of 63.2 weeks. Adverse reactions from the combined double-blind/open-label study data were similar to those observed in the double-blind study.
The following adverse reactions have been reported during clinical trials with the individual components of OPSYNVI but were not observed in 3% or more of subjects treated with OPSYNVI in the A DUE clinical trial:
Macitentan: bronchitis, pharyngitis, transaminases increased, influenza, urinary tract infection.
Tadalafil: lower respiratory tract infection, prolonged erections, gastroesophageal reflux disease, vision blurred, tinnitus, swelling face, chest pain.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during post-approval use of tadalafil. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Macitentan: liver injury, symptomatic hypotension, hypersensitivity reactions (angioedema, pruritus, and rash).
Tadalafil: Cardiovascular and cerebrovascular events including myocardial infarction, sudden cardiac death, stroke, and tachycardia; Nervous system events including, seizure, transient amnesia; Hypersensitivity reactions including urticaria, Stevens-Johnson syndrome, and exfoliative dermatitis; visual field defect, NAION, retinal vascular occlusion; sudden hearing loss, priapism.
-
7 DRUG INTERACTIONS
7.1 Nitrates
Administration of nitrates within 48 hours after the last dose of OPSYNVI is contraindicated [see Contraindications (4.3)] .
7.2 Strong CYP3A4 Inducers
Strong inducers of CYP3A4 such as rifampin significantly reduce macitentan exposure. Use of OPSYNVI with strong CYP3A4 inducers should be avoided [see Clinical Pharmacology (12.3)].
7.3 Strong CYP3A4 Inhibitors
Concomitant use of strong CYP3A4 inhibitors like ketoconazole increase exposure to both macitentan and tadalafil. Avoid concomitant use of OPSYNVI with strong CYP3A4 inhibitors such as ritonavir, ketoconazole and itraconazole. Use other PAH treatment options when strong CYP3A4 inhibitors are needed [see Clinical Pharmacology (12.3)].
7.4 Moderate Dual or Combined CYP3A4 and CYP2C9 Inhibitors
Concomitant use of moderate dual inhibitors of CYP3A4 and CYP2C9 such as fluconazole is predicted to increase macitentan exposure approximately 4-fold. Avoid concomitant use of OPSYNVI with moderate dual inhibitors of CYP3A4 and CYP2C9 (such as fluconazole and amiodarone) [see Clinical Pharmacology (12.3)].
Concomitant treatment of both a moderate CYP3A4 inhibitor and moderate CYP2C9 inhibitor with OPSYNVI should also be avoided [see Clinical Pharmacology (12.3)].
7.5 Alpha-Blockers
PDE5 inhibitors, including tadalafil, and alpha-adrenergic blocking agents are both vasodilators with blood-pressure-lowering effects. In patients who are taking alpha 1blockers, concomitant administration of tadalafil may lead to symptomatic hypotension in some patients. Therefore, the combination of OPSYNVI and doxazosin is not recommended [see Warnings and Precautions (5.3)and Clinical Pharmacology (12.2)].
7.6 Antihypertensives
PDE5 inhibitors, including tadalafil, are mild systemic vasodilators. Clinical pharmacology studies were conducted to assess the effect of tadalafil on the potentiation of the blood–pressure–lowering effects of selected antihypertensive medications (amlodipine, angiotensin II receptor blockers, bendroflumethiazide, enalapril, and metoprolol). Small reductions in blood pressure occurred following coadministration of tadalafil with these agents compared with placebo [see Clinical Pharmacology (12.2)].
7.7 Alcohol
Both alcohol and tadalafil, a PDE5 inhibitor, act as mild vasodilators. When mild vasodilators are taken in combination, blood-pressure–lowering effects of each individual compound may be increased. Substantial consumption of alcohol (e.g., 5 units or greater) in combination with OPSYNVI can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache. Tadalafil (10 mg or 20 mg) did not affect alcohol plasma concentrations and alcohol did not affect tadalafil plasma concentrations [see Clinical Pharmacology (12.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on data from animal reproduction studies, OPSYNVI is contraindicated during pregnancy. Macitentan, a component of OPSYNVI, may cause embryo-fetal toxicity, including birth defects and fetal death, when administered to a pregnant female. Available data from postmarketing reports and published literature over decades of use with ERAs in the same class as OPSYNVI have not identified an increased risk of major birth defects; however, these data are limited. Methodological limitations of these postmarketing reports and published literature include lack of a control group; limited information regarding dose, duration, and timing of drug exposure; and missing data. These limitations preclude establishing a reliable estimate of the risk of adverse fetal and neonatal outcomes with maternal ERA use. Macitentan was teratogenic in rabbits and rats at all doses tested.
Available data from a randomized controlled trial, observational studies, and case series with tadalafil use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In tadalafil animal reproduction studies, no adverse developmental effects were observed with oral administration of tadalafil to pregnant rats and mice during organogenesis at exposures 7 times the maximum recommended human dose (MRHD) of 40 mg/day (see Data) .
There are risks to the mother and the fetus associated with PAH in pregnancy (see Clinical Considerations) . If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, advise pregnant women of the potential risk to a fetus [see Contraindications (4.1)and Warnings and Precautions (5.1)].
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data
Animal Data
Macitentan
In both rabbits and rats, there were cardiovascular and mandibular arch fusion abnormalities. Administration of macitentan to female rats from late pregnancy through lactation caused reduced pup survival and impairment of the male fertility of the offspring at all dose levels tested.
Tadalafil
Tadalafil and/or its metabolites cross the placenta, resulting in fetal exposure in rats.
Animal reproduction studies showed no evidence of teratogenicity, embryotoxicity, or fetotoxicity when tadalafil was given to pregnant rats or mice at unbound tadalafil exposures up to 7 times the exposure at the maximum recommended human dose (MRHD) of 40 mg/day during organogenesis based on AUC. In one of two perinatal/postnatal developmental studies in rats, a reduction of postnatal pup survival was observed at dose levels of 60, 200 and 1000 mg/kg. The no-observed-effect-level (NOEL) for developmental toxicity was 30 mg/kg, which provided maternal exposure to unbound tadalafil concentrations approximately 5 times the exposure at the MRHD based on AUC. Signs of maternal toxicity occurred at doses greater than 200 mg/kg/day, which produced AUCs greater than 8 times the exposure at the MRHD. Surviving offspring had normal development and reproductive performance.
8.2 Lactation
Risk Summary
There are no data on the presence of tadalafil, macitentan, and/or their metabolites in human milk, the effects on the breastfed infant, or the effect on milk production. Tadalafil and/or its metabolites are present in the milk of lactating rats (see Data) . When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the potential for serious adverse reactions in breastfed infants from OPSYNVI, advise women not to breastfeed during treatment with OPSYNVI.
8.3 Females and Males of Reproductive Potential
Based on data from animal reproductive toxicity studies, OPSYNVI can cause fetal harm, including birth defects and fetal death, when administered to a pregnant patient and is contraindicated during pregnancy [see Contraindications (4.1), and Use in Specific Populations (8.1)].
Pregnancy Testing
Verify that patients who can become pregnant are not pregnant prior to initiating OPSYNVI. The patient should contact their physician immediately for pregnancy testing if onset of menses is delayed or pregnancy is suspected. If the pregnancy test is positive, the physician and patient should discuss the risks to the pregnancy, and the fetus .
Contraception
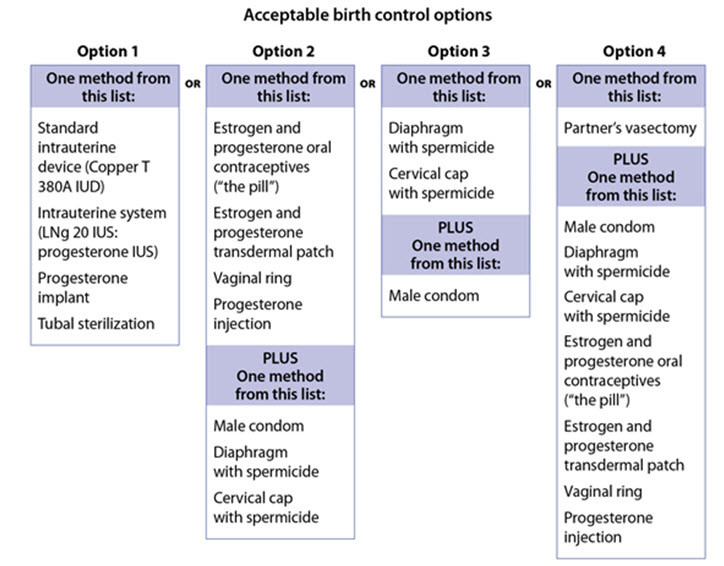
Patients who can become pregnant who are using OPSYNVI should use an effective method of contraception prior to initiation of treatment, during treatment, and for one month after discontinuation of treatment with OPSYNVI to prevent pregnancy [see Warnings and Precautions (5.1)] .
Infertility
Males
Macitentan
Based on findings in animals, macitentan may impair fertility in males of reproductive potential. It is not known whether effects on fertility would be reversible [see Warnings and Precautions (5.10), Clinical Pharmacology (12.2), and Nonclinical Toxicology (13.1)].
Tadalafil
Based on the data from 3 studies in adult males, tadalafil decreased sperm concentrations in the study of 10 mg tadalafil for 6 months and the study of 20 mg tadalafil for 9 months. This effect was not seen in the study of 20 mg tadalafil taken for 6 months. There was no adverse effect of tadalafil 10 mg or 20 mg on mean concentrations of testosterone, luteinizing hormone or follicle stimulating hormone. The clinical significance of the decreased sperm concentrations in the two studies is unknown. There have been no studies evaluating the effect of tadalafil on fertility in men or women [see Clinical Pharmacology (12.2)and Nonclinical Toxicology (13.1)] .
8.5 Geriatric Use
Of the total number of subjects in the clinical study of OPSYNVI for PAH, 20% were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
8.6 Renal Impairment
The use of OPSYNVI is not recommended in patients undergoing dialysis. Avoid use of OPSYNVI in patients with severe renal impairment (creatinine clearance 15–29 mL/min) because of increased tadalafil exposure (AUC), lack of clinical experience and the lack of ability to influence clearance by dialysis. For patients with mild (creatinine clearance 51–80 mL/min) to moderate (creatinine clearance 30–50 mL/min) renal impairment, the recommended dose should be consistent with the adult dosing [see Dosage and Administration (2.1)and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
OPSYNVI was not studied in severe hepatic impairment patients defined as a Model for End-Stage Liver Disease score ≥19. OPSYNVI must not be initiated in patients with severe hepatic impairment, or clinically significant elevated hepatic aminotransferases (greater than 3 times the Upper Limit of Normal at baseline (> 3 × ULN). For patients with mild to moderate hepatic impairment (Child Pugh Class A or B) the recommended dose should be consistent with the adult dosing see Dosage and Administration (2.1)[see Warnings and Precautions (5.2)and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
In the event of an overdose, standard supportive measures should be taken, as required. Dialysis is unlikely to be effective because macitentan is highly protein-bound.
Macitentan
Macitentan has been administered as a single dose of up to and including 600 mg to healthy subjects (60 times the approved dosage). Adverse reactions of headache, nausea and vomiting were observed.
Tadalafil
Single doses of tadalafil up to 500 mg have been given to healthy male subjects, and multiple daily doses up to 100 mg have been given to male patients with erectile dysfunction. Adverse reactions were similar to those seen at lower doses. Doses greater than 40 mg have not been studied in patients with PAH. Hemodialysis contributes negligibly to tadalafil elimination.
-
11 DESCRIPTION
OPSYNVI ® is a single tablet combination containing two oral components used to treat pulmonary arterial hypertension: macitentan, an endothelin receptor antagonist (ERA), and tadalafil, a phosphodiesterase 5 (PDE5) inhibitor.
Macitentan
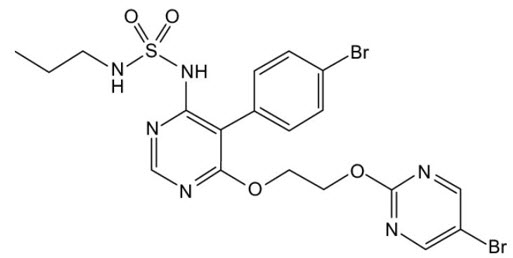
Macitentan is an endothelin receptor antagonist. The chemical name of macitentan is N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4- pyrimidinyl]-N'-propylsulfamide. It has a molecular formula of C 19H 20Br 2N 6O 4S and a molecular weight of 588.27. Macitentan is achiral and has the following structural formula:
Macitentan is a crystalline powder that is insoluble in water. In the solid state macitentan is very stable, is not hygroscopic, and is not light sensitive.
Tadalafil
Tadalafil, an oral treatment for pulmonary arterial hypertension, is a selective inhibitor of cyclic guanosine monophosphate (cGMP)–specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C 22H 19N 3O 4 representing a molecular weight of 389.41. The structural formula is:
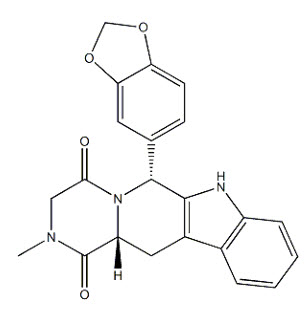
The chemical designation is pyrazino[1´,2´:1,6]pyrido[3,4–b]indole-1,4-dione, 6-(1,3- benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R,12aR)-. It is a crystalline solid that is practically insoluble in water and very slightly soluble in ethanol.
OPSYNVI
OPSYNVI is available as 10 mg/20 mg macitentan/tadalafil and 10 mg/40 mg macitentan/tadalafil film-coated tablets for oral administration.
Each OPSYNVI core tablet contains the following inactive ingredients: Hydroxypropyl cellulose, hydroxypropyl cellulose (low-substituted), lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 80, povidone, sodium starch glycolate, sodium lauryl sulfate.
OPSYNVI 10 mg/20 mg tablets are film-coated with a coating material containing hydroxypropyl methylcellulose, iron oxide red, iron oxide yellow, lactose monohydrate, talc, titanium dioxide, and triacetin.
OPSYNVI 10 mg/40 mg tablets are film-coated with a coating material containing hydroxypropyl methylcellulose, lactose monohydrate, talc, titanium dioxide, and triacetin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Macitentan
Endothelin (ET)-1 and its receptors (ETA and ETB) mediate a variety of deleterious effects, such as vasoconstriction, fibrosis, proliferation, hypertrophy, and inflammation. In disease conditions such as PAH, the local ET system is upregulated and is involved in vascular hypertrophy and in organ damage.
Macitentan is an endothelin receptor antagonist that inhibits the binding of ET-1 to both ETA and ETB receptors. Macitentan displays high affinity and sustained occupancy of the ET receptors in human pulmonary arterial smooth muscle cells. One of the metabolites of macitentan is also pharmacologically active at the ET receptors and is estimated to be about 20% as potent as the parent drug in vitro. The clinical impact of dual endothelin blockage is unknown.
Tadalafil
Tadalafil is an inhibitor of phosphodiesterase type 5 (PDE5), the enzyme responsible for the degradation of cyclic guanosine monophosphate (cGMP). PAH is associated with impaired release of nitric oxide by the vascular endothelium and consequent reduction of cGMP concentrations in the pulmonary vascular smooth muscle. PDE5 is the predominant phosphodiesterase in the pulmonary vasculature. Inhibition of PDE5 by tadalafil increases the concentrations of cGMP resulting in relaxation of pulmonary vascular smooth muscle cells and vasodilation of the pulmonary vascular bed.
Studies in vitrohave shown that tadalafil is a selective inhibitor of PDE5. PDE5 is an enzyme found in corpus cavernosum, vascular smooth muscle, visceral smooth muscle, skeletal muscle, platelets, kidney, lung, and cerebellum. The effect of tadalafil is more potent on PDE5 than on other phosphodiesterases. Tadalafil is > 10,000-fold more potent for PDE5 than for PDE1, PDE2, PDE4 and PDE7, enzymes which are found in the heart, brain, blood vessels, liver, and other organs. Tadalafil is > 10,000-fold more potent for PDE5 than for PDE3, an enzyme found in the heart and blood vessels. This selectivity for PDE5 over PDE3 is important because PDE3 is an enzyme involved in cardiac contractility. Additionally, tadalafil is approximately 700-fold more potent for PDE5 than for PDE6, an enzyme which is found in the retina and is responsible for phototransduction. Tadalafil is also > 9,000-fold more potent for PDE5 than for PDE8, PDE9 and PDE10.
12.2 Pharmacodynamics
Pharmacodynamic studies with OPSYNVI have not been conducted. As OPSYNVI contains macitentan and tadalafil, the pharmacodynamic effects for each component should be considered.
Pulmonary Hemodynamics of Macitentan
The SERAPHIN clinical efficacy study in patients with PAH assessed hemodynamic parameters in a subset of patients after 6 months of treatment. Patients treated with macitentan 10 mg (N=57) achieved a median reduction of 37% (95% CI 22–49) in pulmonary vascular resistance and an increase of 0.6 L/min/m 2 (95% CI 0.3–0.9) in cardiac index compared to placebo (N=67).
Effects on Cardiac Electrophysiology
Effects on Blood Pressure When Administered with Nitrates
In clinical pharmacology studies, tadalafil (5 to 20 mg) was shown to potentiate the hypotensive effect of nitrates. Do not use OPSYNVI in patients taking any form of nitrates [see Contraindications (4.3)].
A double–blind, placebo–controlled, crossover study in 150 male subjects at least 40 years of age (including subjects with diabetes mellitus and/or controlled hypertension) assessed the interaction between nitroglycerin and tadalafil. Subjects received daily doses of tadalafil 20 mg or matching placebo for 7 days and then were given a single dose of 0.4 mg sublingual nitroglycerin (NTG) at pre–specified timepoints following their last dose of tadalafil (2, 4, 8, 24, 48, 72, and 96 hours after tadalafil). A significant interaction between tadalafil and NTG was observed at each timepoint up to and including 24 hours. At 48 hours, by most hemodynamic measures, the interaction between tadalafil and NTG was not observed, although a few more tadalafil subjects compared to placebo experienced greater blood-pressure-lowering effects at this timepoint. After 48 hours, the interaction was not detectable [see Contraindications (4.3)].
Effects on Vision
Single oral doses of PDE inhibitors have demonstrated transient dose-related impairment of color discrimination (blue/green), using the Farnsworth–Munsell 100–hue test, with peak effects near the time of peak plasma levels. This finding is consistent with the inhibition of PDE6, which is involved in phototransduction in the retina. In a study to assess the effects of a single dose of tadalafil 40 mg on vision (N=59), no effects were observed on visual acuity, intraocular pressure, or pupillometry. Across all clinical studies with tadalafil, reports of changes in color vision were rare (<0.1% of patients).
Dose-Response Relationship
Dose-response relationships, between 20 mg and 40 mg of tadalafil, were not observed for 6-minute walk distance or pulmonary vascular resistance (PVR) in subjects with PAH in the placebo-controlled study. Median change from baseline in 6-minute walk distance was 32 meters and 35 meters at 16 weeks in subjects receiving 20 mg and 40 mg daily, respectively. Mean change from baseline PVR was -254 dynes*sec*cm and -209 dynes*sec*cm at 16 weeks in patients receiving 20 mg and 40 mg daily, respectively.
12.3 Pharmacokinetics
Macitentan
The pharmacokinetics of macitentan and its active metabolite have been studied primarily in healthy subjects. The pharmacokinetics of macitentan are dose proportional over a range from 1 mg to 30 mg after once daily administration.
A cross study comparison shows that the exposures to macitentan and its active metabolite in patients with PAH are similar to those observed in healthy subjects.
Tadalafil
Over a dose range of 2.5 to 20 mg, tadalafil exposure (AUC) increases proportionally with dose in healthy subjects. In PAH patients administered between 20 and 40 mg of tadalafil, an approximately 50% greater AUC was observed indicating a less than proportional increase in exposure over the entire dose range of 2.5 to 40 mg.
During tadalafil 20 and 40 mg once daily dosing, steady-state plasma concentrations were attained within 5 days, and exposure was approximately 30% higher than after a single dose.
Absorption
Macitentan
After a single oral administration of OPSYNVI, the maximum plasma concentration of macitentan is achieved in about 10 hours. The absolute bioavailability after oral administration is not known. In a study in healthy subjects, the exposure to macitentan and its active metabolite were unchanged after a high fat breakfast. OPSYNVI may therefore be taken with or without food.
Tadalafil
After a single oral-dose administration of OPSYNVI, the maximum observed plasma concentration (C max) of tadalafil is achieved in about 3 hours. Absolute bioavailability of tadalafil following oral dosing has not been determined. Food intake does not significantly alter the rate and extent of absorption of tadalafil and OPSYNVI may be taken with or without food.
Distribution
Macitentan
Macitentan and its active metabolite are highly bound to plasma proteins (>99%), primarily to albumin and to a lesser extent to alpha-1-acid glycoprotein. The apparent volumes of distribution (Vss/F) of macitentan and its active metabolite were about 50 L and 40 L respectively in healthy subjects.
Metabolism
Macitentan
Following oral administration, the apparent elimination half-lives of macitentan and its active metabolite are approximately 16 and 48 hours, respectively. Macitentan is metabolized primarily by oxidative depropylation of the sulfamide to form the pharmacologically active metabolite. This reaction is dependent on the cytochrome P450 (CYP) system, mainly CYP3A4 with minor contributions of CYP2C8, CYP2C9, and CYP2C19. At steady state in PAH patients, the systemic exposure to the active metabolite is 3-times the exposure to macitentan and is expected to contribute approximately 40% of the total pharmacologic activity.
Tadalafil
Tadalafil is predominantly metabolized by CYP3A to a catechol metabolite. The catechol metabolite undergoes extensive methylation and glucuronidation to form the methylcatechol and methylcatechol glucuronide conjugate, respectively. The major circulating metabolite is the methylcatechol glucuronide. Methylcatechol concentrations are less than 10% of glucuronide concentrations. In vitrodata suggests that metabolites are not expected to be pharmacologically active at observed metabolite concentrations.
Elimination
Macitentan
In a study in healthy subjects with radiolabeled macitentan, approximately 50% of radioactive drug material was eliminated in urine but none was in the form of unchanged drug or the active metabolite. About 24% of the radioactive drug material was recovered from feces.
Tadalafil
Following 40 mg, the mean oral clearance for tadalafil is 3.4 L/hr and the mean effective half-life is 11 hours in healthy subjects. In patients with pulmonary hypertension not receiving concomitant bosentan, the mean oral clearance for tadalafil is 1.6 L/hr. Tadalafil is excreted predominantly as metabolites, mainly in the feces (approximately 61% of the dose) and to a lesser extent in the urine (approximately 36% of the dose).
Population Pharmacokinetics
Tadalafil
In patients with pulmonary hypertension not receiving concomitant bosentan, the average tadalafil exposure at steady-state following 40 mg was 26% higher when compared to those of healthy volunteers. The results suggest a lower clearance of tadalafil in patients with pulmonary hypertension compared to healthy volunteers.
Specific Populations
Renal Impairment
Macitentan
Exposure to macitentan and its active metabolite in patients with severe renal impairment (creatinine clearance 15–29 mL/min) compared to healthy subjects was increased by 30% and 60%, respectively. This increase is not considered clinically relevant.
Tadalafil
In clinical pharmacology studies using single-dose tadalafil (5 to 10 mg), tadalafil exposure (AUC) doubled in subjects with mild (creatinine clearance 51 to 80 mL/min) or moderate (creatinine clearance 30 to 50 mL/min) renal impairment. In subjects with end-stage renal disease on hemodialysis, C max doubled and AUC was 2.7 to 4.1 times as high following single-dose administration of 10 or 20 mg tadalafil, respectively. Exposure to total methylcatechol (unconjugated plus glucuronide) was 2 to 4-times as high in subjects with renal impairment, compared to those with normal renal function. Hemodialysis (performed between 24-and 30-hours post-dose) contributed negligibly to tadalafil or metabolite elimination.
Hepatic Impairment
Macitentan
Exposure to macitentan was decreased by 21%, 34%, and 6% and exposure to the active metabolite was decreased by 20%, 25%, and 25% in subjects with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, and C), respectively. This decrease is not considered clinically relevant.
Tadalafil
In clinical pharmacology studies, tadalafil exposure (AUC) in subjects with mild or moderate hepatic impairment (Child-Pugh Class A or B) was similar to exposure in healthy subjects when a dose of 10 mg was administered. There are no available data for doses higher than 10 mg of tadalafil in patients with hepatic impairment. Insufficient data are available for subjects with severe hepatic impairment (Child-Pugh Class C) .
Geriatric Patients
Drug Interactions
No clinical study evaluating drug interactions has been performed using OPSYNVI. Interactions that have been identified in studies with individual components of OPSYNVI (macitentan or tadalafil) determine the interactions that may occur with OPSYNVI. Co-administration of macitentan (10 mg once daily) and tadalafil (40 mg once daily) had no clinically relevant effect on the pharmacokinetics of either macitentan or tadalafil.
Macitentan
The metabolism of macitentan to its active metabolite is catalyzed mainly by CYP3A4, with minor contributions from CYP2C8, CYP2C9, and CYP2C19.
Macitentan and its active metabolite do not have relevant inhibitory or inducing effects on CYP enzymes.
Macitentan and its active metabolite are not substrates of the multi-drug resistance protein (P-gp, MDR-1) or organic anion transporting polypeptides (OATP1B1 and OATP1B3).
Macitentan and its active metabolite are not inhibitors of hepatic or renal drug transporters at clinically relevant concentrations.
Effects of other drugs on macitentan or tadalafil
Strong CYP3A4 inducers or inhibitors
Macitentan
Concomitant treatment with rifampicin 600 mg daily, a potent inducer of CYP3A4, reduced the steady-state exposure to macitentan by 79% but did not affect the exposure to the active metabolite [see Drug Interactions (7.2)].
Concomitant use of strong CYP3A4 inhibitors like ketoconazole approximately double macitentan exposure. Effects of other strong CYP3A4 inhibitors such as ritonavir on macitentan were not studied but are likely to result in an increase in macitentan exposure at steady state similar to that seen with ketoconazole [see Drug Interactions (7.3)] .
A moderate dual inhibitor of CYP3A4 and CYP2C9 such as fluconazole (400 mg once daily) is predicted to increase macitentan exposure approximately 4-fold without relevant effect on the exposure to its active metabolite [see Drug Interactions (7.4)] .
Tadalafil
Rifampin (600 mg daily), a CYP3A inducer, reduced tadalafil 10 mg single-dose exposure (AUC) by 88% and C max by 46%, relative to the values for tadalafil 10 mg alone [see Drug Interactions (7.2)] .
Bosentan, a substrate of CYP2C9 and CYP3A and a moderate inducer of CYP3A, CYP2C9 and possibly CYP2C19, reduced tadalafil systemic exposure following multiple-dose coadministration. Although specific interactions have not been studied, other CYP3A inducers, such as carbamazepine, phenytoin, and phenobarbital, would likely decrease tadalafil exposure [see Drug Interactions (7.2)].
Ketoconazole increased tadalafil exposure relative to the values for tadalafil alone. Although specific interactions have not been studied, other CYP3A inhibitors, such as erythromycin, itraconazole, and grapefruit juice, would likely increase tadalafil exposure [see Drug Interactions (7.3)].
Ritonavir increased tadalafil 20-mg single-dose exposure relative to the values for tadalafil alone. Ritonavir inhibits and induces CYP3A, the enzyme involved in the metabolism of tadalafil, in a time-dependent manner. The initial inhibitory effect of ritonavir on CYP3A may be mitigated by a more slowly evolving induction effect so that after about 1 week of ritonavir twice daily, the exposure of tadalafil is similar in the presence of and absence of ritonavir [see Drug Interactions (7.3)] .
Effects of macitentan or tadalafil on other medicinal products
Macitentan
Macitentan once daily dosing did not alter the exposure to R- and S-warfarin or their effect on international normalized ratio (INR).
At steady state, the exposure to sildenafil 20 mg t.i.d. increased by 15% during concomitant administration of macitentan 10 mg once daily. This change is not considered clinically relevant. Macitentan 10 mg once daily did not affect the pharmacokinetics of an oral contraceptive (norethisterone 1 mg and ethinyl estradiol 35 µg).
Macitentan 10 mg once daily did not affect the pharmacokinetics of concomitant use of a BCRP substrate drug (riociguat 1 mg and rosuvastatin 10 mg).
Tadalafil
Tadalafil is not expected to cause clinically significant inhibition or induction of the clearance of drugs metabolized by cytochrome P450 (CYP) isoforms.
Exposure changes of drugs following co-administration with tadalafil are shown in Figure 1.
Figure 1: Impact of Tadalafil on the Pharmacokinetics of Other Drugs
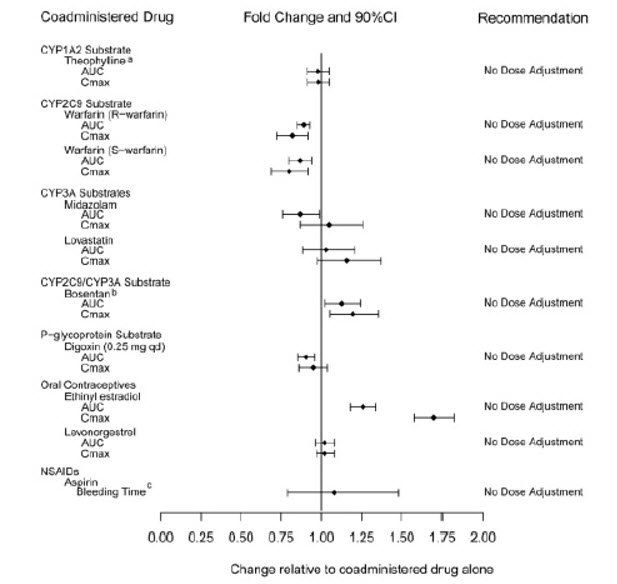
aA small augmentation (increase of 3 beats per minute) in heart rate was observed with theophylline.
bTadalafil (40 mg qd) had no clinically significant effect on exposure (AUC and C) of bosentan
metabolites.
c95% CI -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Macitentan
Carcinogenicity studies of 2 years' duration did not reveal any carcinogenic potential at exposures 75-fold and 140-fold the human exposure (based on AUC) in male and female mice, respectively, and 8.3-and 42-fold in male and female rats, respectively.
Tadalafil
Tadalafil was not carcinogenic to rats or mice when administered daily for 2 years at doses up to 400 mg/kg/day. Systemic drug exposures, as measured by AUC of unbound tadalafil, were approximately 5-fold for mice, and 7-and 14-fold for male and female rats, respectively, the exposures at the maximum recommended human dose (MRHD) of 40 mg.
Mutagenesis
Impairment of Fertility
Macitentan
Treatment of juvenile rats from postnatal Day 4 to Day 114 led to reduced body weight gain and testicular tubular atrophy at exposures 7-fold the human exposure. Fertility was not affected.
Reversible testicular tubular dilatation was observed in chronic toxicity studies at exposures greater than 7-fold and 23-fold the human exposure in rats and dogs, respectively. After 2 years of treatment, tubular atrophy was seen in rats at 4-fold the human exposure. Macitentan did not affect male or female fertility at exposures ranging from 19-to 44-fold the human exposure, respectively, and had no effect on sperm count, motility, and morphology in male rats. No testicular findings were noted in mice after treatment up to 2 years.
Tadalafil
There were no effects on fertility, reproductive performance or reproductive organ morphology in male or female rats given oral doses of tadalafil up to 400 mg/kg/day, a dose producing AUCs for unbound tadalafil of 6-fold for males or 17-fold for females the exposures at the MRHD of 40 mg. In beagle dogs given tadalafil daily for 3 to 12 months, there was treatment-related non-reversible degeneration and atrophy of the seminiferous tubular epithelium in the testes in 20–100% of the dogs that resulted in a decrease in spermatogenesis in 40–75% of the dogs at doses of ≥10 mg/kg/day. Systemic exposure (based on AUC) at no-observed-adverse-effect-level (NOAEL) (10 mg/kg/day) for unbound tadalafil was similar to that expected in humans at the MRHD of 40 mg.
There were no treatment-related testicular findings in rats or mice treated with doses up to 400 mg/kg/day for 2 years.
13.2 Animal Toxicology and/or Pharmacology
Macitentan
In dogs, macitentan decreased blood pressure at exposures similar to the therapeutic human exposure. Intimal thickening of coronary arteries was observed at 17-fold the human exposure after 4 to 39 weeks of treatment. Due to the species-specific sensitivity and the safety margin, this finding is considered not relevant for humans.
There were no adverse liver findings in long-term studies conducted in mice, rats and dogs at exposures of 12-to 116-fold the human exposure.
Tadalafil
Animal studies showed vascular inflammation in tadalafil-treated mice, rats, and dogs. In mice and rats, lymphoid necrosis and hemorrhage were seen in the spleen, thymus, and mesenteric lymph nodes at unbound tadalafil exposure of 1-to 17-fold the human exposure (AUCs) at the MRHD of 40 mg. In dogs, an increased incidence of disseminated arteritis was observed in 1-and 6-month studies at unbound tadalafil exposure of 0.5-to 38-fold the human exposure (AUC) at the MRHD of 40 mg. In a 12-month dog study, no disseminated arteritis was observed, but 2 dogs exhibited marked decreases in white blood cells (neutrophils) and moderate decreases in platelets with inflammatory signs at unbound tadalafil exposures of approximately 4-to 10-fold the human exposure at the MRHD of 40 mg. The abnormal blood-cell findings were reversible within 2 weeks upon removal of the drug.
-
14 CLINICAL STUDIES
14.1 Pulmonary Arterial Hypertension
OPSYNVI
OPSYNVI was demonstrated to reduce pulmonary vascular resistance (PVR) in a multi-national, multi-center, double-blind, adaptive, randomized, active-controlled, parallel-group study [NCT03904693 (A DUE)] in 187 patients with PAH (WHO FC II–III). The study was designed to compare the efficacy and safety of OPSYNVI to each monotherapy macitentan or tadalafil. Patients with PVR of at least 240 dyn∙s/cm 5 were randomized to receive OPSYNVI (n=108), 10 mg macitentan monotherapy (n=35) or 40 mg tadalafil monotherapy (n=44), once daily.
Patients who received treatment during the double-blind treatment period (n=186) were either treatment-naïve (53%) or on an ERA (17%) or a PDE5 inhibitor (30%). Patients enrolled had idiopathic PAH (51%), heritable PAH (5%), PAH associated with connective tissue disease (35%), or PAH associated with congenital heart disease (3%). The mean age was 50 years (range 18–80), 20% of patients were ≥65 years of age, 22% were male and 62% were white. At the time of enrollment, 51% of patients were WHO FC II and 49% were WHO FC III.
Patients who were not on a therapeutic PDE5 inhibitor dose at baseline received a 1-week titration period of macitentan 10 mg and tadalafil 20 mg.
The primary endpoint of the study was change from baseline in PVR (expressed as the ratio of geometric means of end of double-blind treatment to baseline) vs the individual component monotherapies after 16 weeks.
Hemodynamic
OPSYNVI demonstrated greater reduction in PVR after 16 weeks. Treatment with OPSYNVI resulted in a statistically significant treatment effect of 0.71 (95% CL 0.61, 0.82, p < 0.0001) representing a 29% reduction in PVR as compared to macitentan, and of 0.72 (95% CLs 0.64, 0.80, p < 0.0001) representing a 28% reduction in PVR as compared to tadalafil (Table 4).
Table 4: Change from Baseline in PVR at Week 16 Treatment-naïve and Prior ERA Treatment Treatment-naïve and Prior PDE5 Inhibitor Treatment Macitentan
(n=35)OPSYNVI
(n=70)Tadalafil
(n=44)OPSYNVI
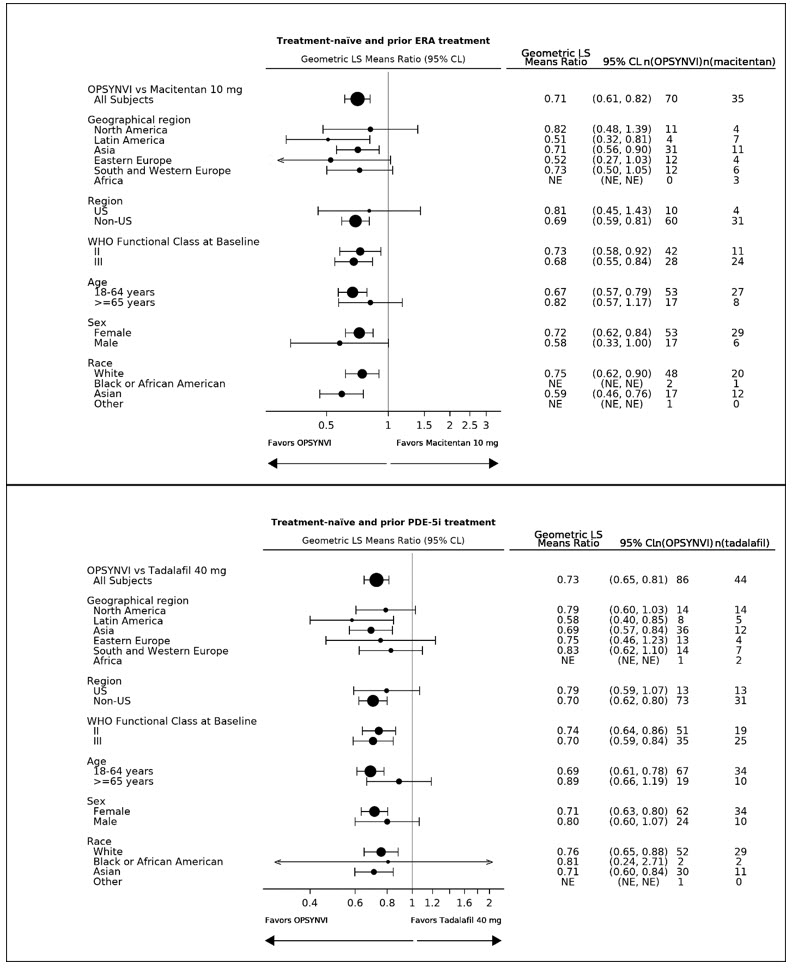
(n=86)Baseline mean (SD) 816 (401) 834 (631) 802 (552) 885(640) Reduction at Week 16 (dynes *sec/cm5) Mean (SD) -162 (240) -371 (429) -181 (238) -385 (396) Geometric mean (Week 16/Baseline) 0.77 0.55 0.78 0.56 Treatment effect ratios (95% CL) -29% (-39%, -18%) -28% (-36%, -20%) 2-sided p-value <0.0001 <0.0001 A consistent effect of OPSYNVI on reducing PVR was seen across subgroups of age, sex, race, geographical region, and baseline WHO FC (see Figure 2). Additionally, a consistent effect was observed in patients who were either treatment-naïve, or previously exposed to an ERA or PDE5 inhibitor.
Figure 2: Subgroup Analysis of the A DUE Study
The individual components of OPSYNVI, macitentan and tadalafil, have been approved and used independently or concomitantly in the clinical setting to effectively manage PAH. Macitentan is an endothelin receptor antagonist (ERA) indicated for the treatment of pulmonary arterial hypertension (PAH, WHO Group I) to reduce the risks of disease progression and hospitalization for PAH. Tadalafil is a phosphodiesterase 5 (PDE5) inhibitor indicated for the treatment of pulmonary arterial hypertension (PAH, WHO Group 1) to improve exercise ability.
Macitentan
The primary efficacy endpoint of the multi-center, long-term, placebo-controlled SERAPHIN study was time to the first occurrence of death, a significant morbidity event, defined as atrial septostomy, lung transplantation, initiation of intravenous or subcutaneous prostanoids, or "other worsening of PAH" during double-blind treatment plus 7 days. Other worsening was defined as all of the following: 1) a sustained ≥15% decrease from baseline in 6-minute walk distance (6MWD), 2) worsening of PAH symptoms (worsening of WHO FC), and 3) need for additional treatment for PAH. All of these other worsening events were confirmed by an independent adjudication committee, blinded to treatment allocation.
Treatment with OPSUMIT 10 mg reduced the risk of clinical worsening events and hospitalization for PAH.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
OPSYNVI ® (macitentan and tadalafil) tablets, 10 mg/20 mg, are supplied as pink, oblong film-coated tablets debossed with "1020" on one side and "MT" on the other side.
OPSYNVI 10 mg/20 mg is supplied as follows:
7-count bottles with child-resistant closure NDC 66215-812-07
7-count blister NDC 66215-812-08
30-count bottle with child-resistant closure NDC 66215-812-30OPSYNVI ® (macitentan and tadalafil) tablets, 10 mg/40 mg, are supplied as white to almost-white, oblong film-coated tablets debossed with "1040" on one side and "MT" on the other side.
OPSYNVI 10 mg/40 mg is supplied as follows:
10-count blister NDC 66215-814-10
30-count bottle with child-resistant closure NDC 66215-814-30 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Embryo-Fetal Toxicity
Counsel female patients of reproductive potential about the need to use effective methods of contraception prior to initiation of treatment with OPSYNVI, during treatment, and for one month after treatment discontinuation. Females of reproductive potential should have a negative pregnancy test prior to treatment with OPSYNVI [see Contraindications (4.1), and Use in Specific Populations (8.1, 8.3)] .
Patients should be instructed to contact their physician if they suspect they may be pregnant. Patients should seek additional contraceptive advice from a gynecologist or similar expert as needed.
Educate and counsel females of reproductive potential on the use of emergency contraception in the event of unprotected sex or contraceptive failure.
Advise pre-pubertal females to report any changes in their reproductive status immediately to her prescriber.
Review the Medication Guide with female patients.
Lactation
Advise women not to breastfeed during treatment with OPSYNVI [see Use in Specific Populations (8.2)] .
Infertility
Advise males of reproductive potential that OPSYNVI may impair fertility [see Warnings and Precautions (5.10), Clinical Pharmacology (12.2), Use in Specific Populations (8.3), and Nonclinical Toxicology (13.1)].
Hepatotoxicity
Some members of this pharmacological class are hepatotoxic. Educate patients on signs of hepatotoxicity. Advise patients that they should contact their doctor if they have unexplained nausea, vomiting, right upper quadrant pain, fatigue, anorexia, jaundice, dark urine, fever, or itching.
Hemoglobin Decrease
Advise patients that they may develop anemia. Advise patients that they will have blood tests to check their red blood cells before starting OPSYNVI.
Use with Organic Nitrates or Guanylate Cyclase (GC) Stimulators
Inform patients of contraindication of OPSYNVI with any use of organic nitrates or GC stimulators.
Vision Loss
Advise patients to seek immediate medical attention in the event of a sudden loss of vision in one or both eyes while taking OPSYNVI. Such an event may be a sign of NAION. Also discuss with patients that there is an increased risk of NAION in individuals who have already experienced NAION in one eye, including whether such individuals could be adversely affected by use of vasodilators such as PDE5 inhibitors.
Hearing Loss
Advise patients to seek prompt medical attention in the event of sudden decrease or loss of hearing while taking OPSYNVI. These events may be accompanied by tinnitus and dizziness.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
OPSYNVI ® (op-SIN-vee)
(macitentan and tadalafil)
tabletsThis Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 4/2025 Read this Medication Guide for OPSYNVI before you start taking OPSYNVI and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or your treatment. What is the most important information I should know about OPSYNVI?
- Serious birth defects.
- Females should not be pregnant when they start taking OPSYNVI or become pregnant during treatment with OPSYNVI.
- Females who are able to get pregnant should have a negative pregnancy test before beginning treatment with OPSYNVI.
Talk to your healthcare provider about your menstrual cycle. Your healthcare provider will decide when to do the pregnancy test and will order a pregnancy test for you depending on your menstrual cycle.- Females who
are ableto get pregnant are females who:
- have entered puberty, even if they have not started their menstrual period, and
- have a uterus, and
- have not gone through menopause. Menopause means that you have not had a menstrual period for at least 12 months for natural reasons, or that you have had your ovaries removed.
- Females who
are not ableto get pregnant are females who:
- have not yet entered puberty, or
- do not have a uterus, or
- have gone through menopause. Menopause means that you have not had a menstrual period for at least 12 months for natural reasons, or that you have had your ovaries removed, or
- are infertile for other medical reasons and this infertility is permanent and cannot be reversed.
- Females who
are ableto get pregnant are females who:
- Talk with your healthcare provider or gynecologist (a doctor who specializes in female reproduction) to find out about options for acceptable birth control that you may use to prevent pregnancy during treatment with OPSYNVI.
- If you decide that you want to change the form of birth control that you use, talk with your healthcare provider or gynecologist to be sure that you choose another acceptable form of birth control.
- Do not have unprotected sex.Talk to your healthcare provider or pharmacist right away if you have unprotected sex or if you think your birth control has failed. Your healthcare provider may talk with you about using emergency birth control.
- Tell your healthcare provider right away if you miss a menstrual period or think you may be pregnant.
If you are a female who can get pregnant, you should talk to your healthcare provider to understand the benefits and risks of OPSYNVI.
See " What are the possible side effects of OPSYNVI?" for more information about side effects.What is OPSYNVI? - OPSYNVI is a prescription medicine that contains 2 medicines called macitentan and tadalafil. OPSYNVI is used for long-term treatment of adults with pulmonary arterial hypertension (PAH), which is high blood pressure in the arteries of your lungs.
- It is not known if OPSYNVI is safe and effective in children.
Who should not take OPSYNVI?
Do not take OPSYNVI if you:- are pregnant, plan to become pregnant, or become pregnant during treatment with OPSYNVI. OPSYNVI can cause serious birth defects (see " What is the most important information I should know about OPSYNVI?")
- are allergic to macitentan, tadalafil, or any of the ingredients in OPSYNVI. See the end of this Medication Guide for a complete list of ingredients in OPSYNVI.
- take any medicines called nitrates
- take any medicines called guanylate cyclase (GC) stimulators
Before taking OPSYNVI, tell your healthcare provider about all of your medical conditions, including if you: - have liver problems
- have low blood pressure
- have anemia
- have heart problems including heart attack or heart failure
- have pulmonary veno-occlusive disease (PVOD)
- have any eye problems, including NAION or an inherited eye disorder called retinitis pigmentosa
- have hearing problems such as ringing in the ears, dizziness, or loss of hearing
- have a deformed penis shape or Peyronie's disease or have blood cell problems such as sickle cell anemia, multiple myeloma, or leukemia. These conditions increase your risk of getting a prolonged erection.
- have kidney problems or get dialysis
- are pregnant or plan to become pregnant during OPSYNVI treatment. OPSYNVI can cause serious birth defects. See " What is the most important information I should know about OPSYNVI?"
- are breastfeeding or plan to breast feed. It is not known if OPSYNVI passes into your breastmilk. Do notbreastfeed during treatment with OPSYNVI.
Especially tell your healthcare provider if you take:- nitrate medicines
- HIV medicines
- antifungal medicines
- antiseizure medicines
- medicines used to treat infection
- erectile dysfunction medicines
- blood pressure medicines
- medicines used to treat PAH or heart problems
Ask your healthcare provider or pharmacist if you are not sure if your medicine is one that is listed above. Know the medicines you take. Keep a list and show it to your healthcare provider or pharmacist when you get a new medicine. How should I take OPSYNVI?
OPSYNVI will be mailed to you by a specialty pharmacy. Your healthcare provider will give you complete details.- Take OPSYNVI exactly as your healthcare provider tells you to take it. Do notstop taking OPSYNVI unless your healthcare provider tells you.
- Take OPSYNVI with or without food.
- Do notcut, crush, or chew OPSYNVI tablets.
- If you take too much OPSYNVI, call your healthcare provider or go to the nearest hospital emergency room right away.
- If you miss a dose of OPSYNVI, take it as soon as you remember that day. Take the next dose at your regular time. Do nottake 2 doses at the same time to make up for a missed dose.
What should I avoid while taking OPSYNVI? - Do nothave more than 4 alcohol-containing drinks in a short period of time during treatment with OPSYNVI. Drinking too much alcohol can increase your chances of getting low blood pressure, increased heart rate, dizziness, and headache.
What are the possible side effects of OPSYNVI?
OPSYNVI can cause serious side effects, including:- Serious birth defects.See " What is the most important information I should know about OPSYNVI?"
- Liver problems. OPSYNVI can cause liver problems. Your healthcare provider should do blood tests to check your liver before you start taking OPSYNVI. Tell your healthcare provider if you have any of the following symptoms of liver problems during treatment with OPSYNVI.
- nausea or vomiting
- pain in the upper right stomach
- tiredness
- loss of appetite
- yellowing of your skin or whites of your eyes
- dark urine
- fever
- itching
- Decreased blood pressure (hypotension).OPSYNVI can cause low blood pressure that lasts for a short time.
- Low red blood cell levels (anemia) can occur with OPSYNVI treatment, usually during the first weeks after starting therapy.Your healthcare provider will do blood tests to check your red blood cells before starting and as needed during treatment with OPSYNVI.
- Vision loss.OPSYNVI can cause decreased eyesight or permanent loss of vision in 1 or both eyes, which could be a sign of NAION. There is an increased risk of NAION in people who have already had NAION in 1 eye. If you notice a sudden decrease or loss of vision in 1 or both eyes, contact your healthcare provider right away.
- Hearing problems.Sudden decrease or loss of hearing, sometimes with ringing in the ears and dizziness, can happen during treatment with OPSYNVI. If you notice a sudden decrease or loss of hearing, contact your healthcare provider right away.
- Fluid retention.Fluid retention can happen within weeks after starting OPSYNVI and could lead to hospitalization. Tell your healthcare provider right away if you have any unusual weight gain, trouble breathing, or swelling of your ankles or legs. Your healthcare provider will look for the cause of any fluid retention and may stop treatment with OPSYNVI.
- Decreased sperm count.OPSYNVI can cause decreased sperm counts in males and may affect the ability to father a child. Tell your healthcare provider if being able to have children is important to you.
- Prolonged erection.Erections that last more than 4 hours, with or without pain, can happen during treatment with OPSYNVI. Painful erections (priapism) can cause permanent damage to the penis if not treated right away. Tell your healthcare provider right away if you have an erection that lasts longer than 4 hours, with or without pain.
- too much fluid in your body (fluid retention) and swelling caused by too much fluid (edema)
- anemia
- headache, including migraine headache
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store OPSYNVI? - Store OPSYNVI tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Store OPSYNVI in the package that it comes in to protect from moisture.
- OPSYNVI contains a desiccant to help keep the tablets dry. Do notdiscard or eat the desiccant.
- OPSYNVI bottles have a child-resistant cap.
- Keep OPSYNVI and all medicines out of the reach of children.
General information about the safe and effective use of OPSYNVI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use OPSYNVI for a condition for which it was not prescribed. Do not give OPSYNVI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about OPSYNVI that is written for health professionals.What are the ingredients in OPSYNVI?
Active ingredients:macitentan and tadalafil
Inactive ingredients: tablet core:hydroxypropyl cellulose, hydroxypropyl cellulose (low substituted), lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 80, povidone, sodium starch glycolate, sodium lauryl sulfate. 10 mg/20 mg film-coating:hydroxypropyl methylcellulose, iron oxide red, iron oxide yellow, lactose monohydrate, talc, titanium dioxide, triacetin. 10 mg/40 mg film-coating:hydroxypropyl methylcellulose, lactose monohydrate, talc, titanium dioxide, triacetin.
Manufactured for:
Actelion Pharmaceuticals US, Inc.
a Janssen Pharmaceutical Company
Titusville, NJ 08560, USA
For patent information: www.janssenpatents.com
For more information, call 1-800-526-7736 (1-800-JANSSEN), or visit www.OPSYNVI.com.
© 2024 Actelion Pharmaceuticals US, Inc. - PRINCIPAL DISPLAY PANEL - 10 mg/20 mg Tablet Bottle Carton
- PRINCIPAL DISPLAY PANEL - 10 mg/40 mg Tablet Bottle Carton
-
INGREDIENTS AND APPEARANCE
OPSYNVI
macitentan and tadalafil tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66215-814 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MACITENTAN (UNII: Z9K9Y9WMVL) (MACITENTAN - UNII:Z9K9Y9WMVL) MACITENTAN 10 mg TADALAFIL (UNII: 742SXX0ICT) (TADALAFIL - UNII:742SXX0ICT) TADALAFIL 40 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (white to almost white) Score no score Shape OVAL (oblong) Size 15mm Flavor Imprint Code 1040;MT Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66215-814-30 1 in 1 CARTON 03/22/2024 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:66215-814-10 1 in 1 CARTON 03/22/2024 2 NDC:66215-814-01 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218490 03/22/2024 OPSYNVI
macitentan and tadalafil tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66215-812 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MACITENTAN (UNII: Z9K9Y9WMVL) (MACITENTAN - UNII:Z9K9Y9WMVL) MACITENTAN 10 mg TADALAFIL (UNII: 742SXX0ICT) (TADALAFIL - UNII:742SXX0ICT) TADALAFIL 20 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color pink Score no score Shape OVAL (oblong) Size 13mm Flavor Imprint Code 1020;MT Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66215-812-07 1 in 1 CARTON 03/22/2024 1 7 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:66215-812-30 1 in 1 CARTON 03/22/2024 2 30 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:66215-812-08 1 in 1 CARTON 03/22/2024 3 NDC:66215-812-01 7 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218490 03/22/2024 Labeler - Actelion Pharmaceuticals US, Inc. (002641228) Establishment Name Address ID/FEI Business Operations SMS Pharmaceuticals Limited 650460954 analysis(66215-812, 66215-814) , api manufacture(66215-812, 66215-814) , label(66215-812, 66215-814) , pack(66215-812, 66215-814) Establishment Name Address ID/FEI Business Operations SMS Pharmaceuticals Limited 677605501 analysis(66215-812, 66215-814) Establishment Name Address ID/FEI Business Operations Vimta Labs Limited 675597260 analysis(66215-812, 66215-814) Establishment Name Address ID/FEI Business Operations Lonza AG 480007517 analysis(66215-812, 66215-814) , api manufacture(66215-812, 66215-814) Establishment Name Address ID/FEI Business Operations Jetpharma SA 481885861 api manufacture(66215-812, 66215-814) , particle size reduction(66215-812, 66215-814) Establishment Name Address ID/FEI Business Operations PENN PHARMACEUTICAL SERVICES LIMITED 226277259 analysis(66215-812, 66215-814) , manufacture(66215-812, 66215-814) Establishment Name Address ID/FEI Business Operations Janssen Cilag SpA 542797928 analysis(66215-812, 66215-814) Establishment Name Address ID/FEI Business Operations Almac Pharma Services Limited 233170864 analysis(66215-812, 66215-814) , label(66215-812, 66215-814) , pack(66215-812, 66215-814) Establishment Name Address ID/FEI Business Operations Johnson & Johnson Private Limited 677603030 analysis(66215-812, 66215-814)