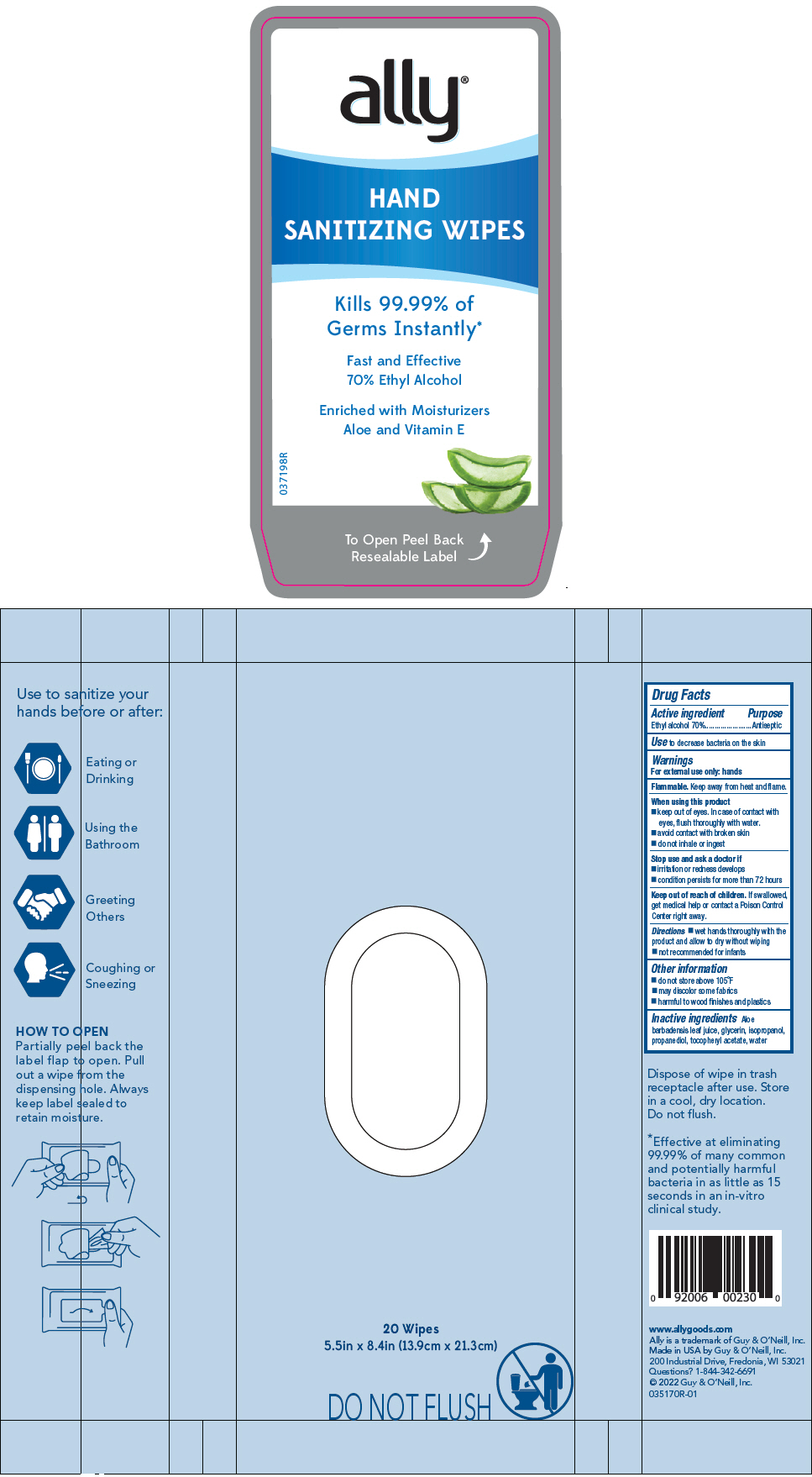
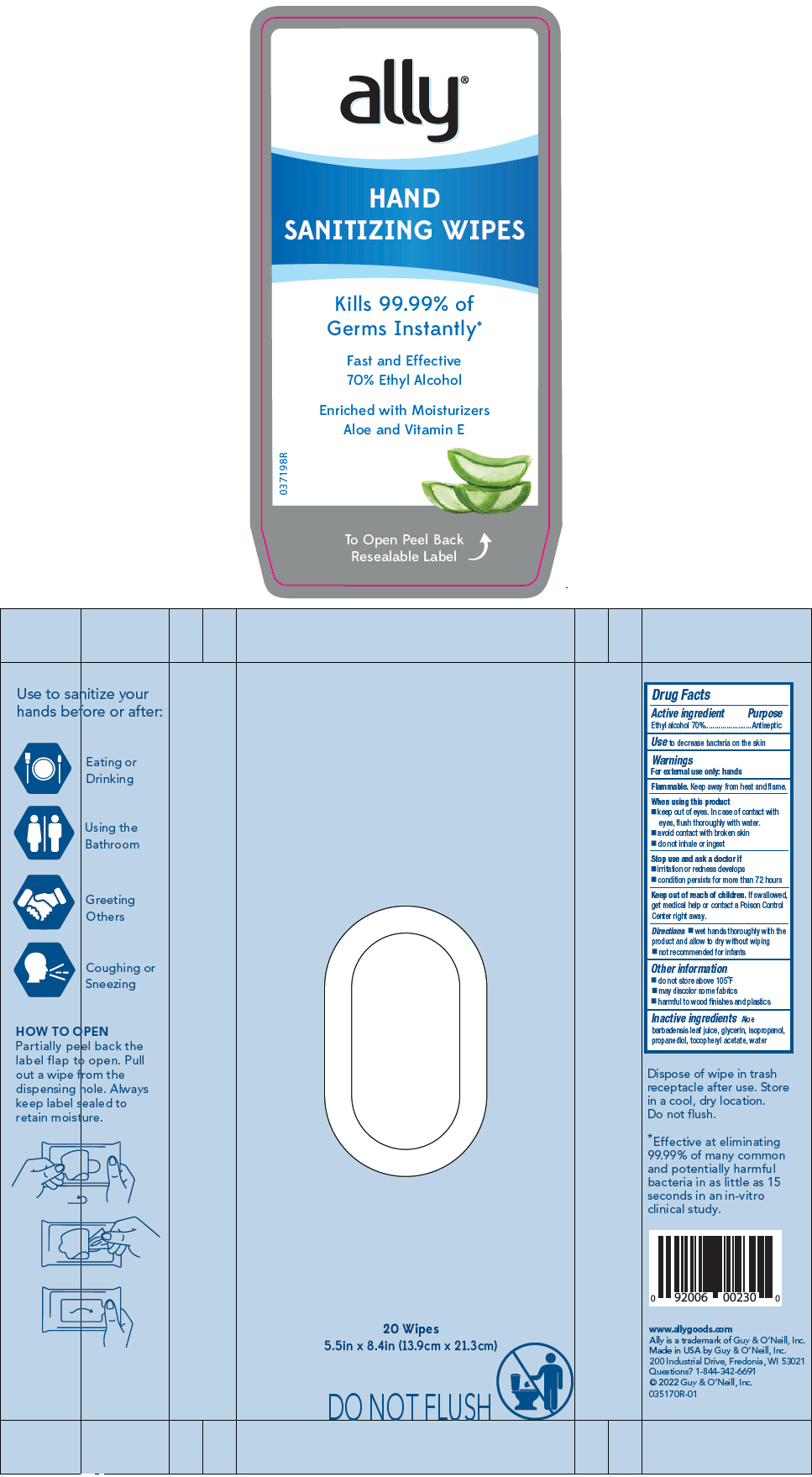
Label: ALLY HAND SANITIZER WIPES- alcohol cloth
- NDC Code(s): 50862-1310-4
- Packager: Guy & O'Neill, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 20 Wipe Pouch
-
INGREDIENTS AND APPEARANCE
ALLY HAND SANITIZER WIPES
alcohol clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50862-1310 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 29 mL ISOPROPYL ALCOHOL (UNII: ND2M416302) 1 mL GLYCERIN (UNII: PDC6A3C0OX) 1 mL PROPANEDIOL (UNII: 5965N8W85T) 0.5 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.1 mL .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 0.001 mL Product Characteristics Color Score no score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50862-1310-4 20 in 1 POUCH; Type 0: Not a Combination Product 04/05/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M003 04/05/2022 Labeler - Guy & O'Neill, Inc. (037838844) Registrant - Guy & O'Neill, Inc. (037838844) Establishment Name Address ID/FEI Business Operations Guy & O'Neill, Inc. 037838844 MANUFACTURE(50862-1310) , LABEL(50862-1310)