Label: CYBERDERM SIMPLY ZINC ULTRA- zinc oxide lotion
- NDC Code(s): 71644-003-01, 71644-003-50, 71644-003-80
- Packager: Cyberderm Laboratories Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
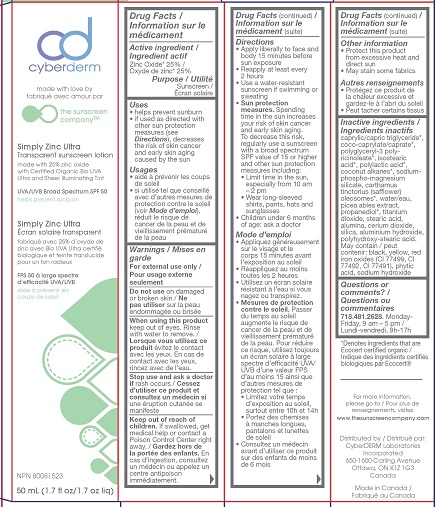
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- Apply liberally to face and body 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
- Children under 6 months of age: ask a doctor
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients caprylic/capric triglyceride*, coco-caprylate/caprate*, polyglyceryl-3 polyricinoleate*, isostearic acid*, polylactic acid*, coconut alkanes*, sodium-phospho-magnesium silicate, carthamus tinctorius (safflower) oleosomes*, water/eau, picea abies extract, propanediol*, titanium dioxide, stearic acid, alumina, cerium dioxide, silica, aluminum hydroxide, polyhydroxystearic acid, May contain black, yellow, red iron oxides (CI 77499, CI 77492, CI 77491), phytic acid, sodium hydroxide.
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CYBERDERM SIMPLY ZINC ULTRA
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71644-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 25 g in 100 mL Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) PICEA ABIES FLOWER BUD (UNII: WQZ50FAU2B) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CERIC OXIDE (UNII: 619G5K328Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FYTIC ACID (UNII: 7IGF0S7R8I) FERRIC OXIDE RED (UNII: 1K09F3G675) COCONUT ALKANES (UNII: 1E5KJY107T) SODIUM HYDROXIDE (UNII: 55X04QC32I) ISOSTEARIC ACID (UNII: X33R8U0062) WATER (UNII: 059QF0KO0R) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) POLYLACTIDE (UNII: 459TN2L5F5) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71644-003-50 1 in 1 CARTON 03/30/2018 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:71644-003-80 1 in 1 CARTON 03/30/2018 2 80 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:71644-003-01 1 in 1 CARTON 06/17/2020 3 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/30/2018 Labeler - Cyberderm Laboratories Inc. (242524267)