Label: TOPICAL PAIN RELIEF- methyl salicylate, menthol, capsaicin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 50436-9990-1 - Packager: Unit Dose Services
- This is a repackaged label.
- Source NDC Code(s): 76074-120
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 15, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
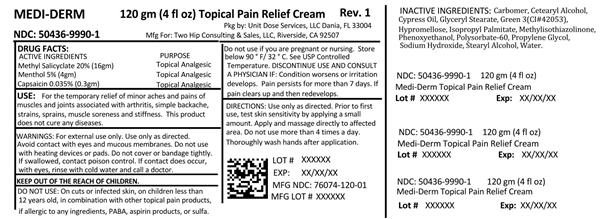
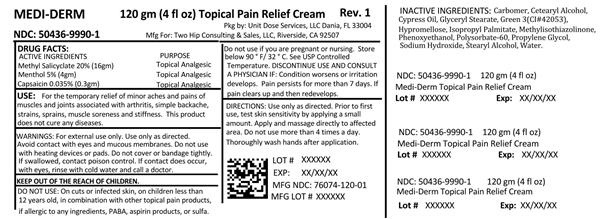
ACTIVE INGREDIENT
Active ingredients Purpose
Methyl salicylate 20% (16gm) Topical Analgesic
Menthol 5% (4gm) Topical Analgesic
Capsaicin 0.035% (0.3gm) Topical Analgesic
For the temporary relief of minor aches and pains of muscles and joints associated with arthritis, simple backache, strains, sprains, muscle soreness and stiffness. This product does not cure any diseases.
Discontinue use and consult a physician if condition worsens or irritation develops. Pain persists for more than 7 days. If pain clears up and then redevelops.
Warnings: For external use only. Use only as directed. Avoid contact with eyes and mucous membranes. Do not use with heating devices or pads. Do not cover or bandage tightly. If swallowed, call poison control. If contact does occur with eyes rinse with cold water and call a doctor. Do not use: on cuts or infected skin, on children less than 12 years old, in combination with other topical pain products, if allergic to any ingredients, PABA, aspirin products, or sulfa. Do not use if you are pregnant or nursing. Store below 90 degrees F/32 degrees C. See USP Controlled Temperature.
- HOW SUPPLIED
- TOPICAL PAIN RELIEF (METHYL SALICYLATE, MENTHOL, CAPSAICIN) CREAM
-
INGREDIENTS AND APPEARANCE
TOPICAL PAIN RELIEF
methyl salicylate, menthol, capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50436-9990(NDC:76074-120) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 20 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g in 100 g CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN .0355 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) HYPROMELLOSE 2208 (100000 MPA.S) (UNII: VM7F0B23ZI) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 60 (UNII: CAL22UVI4M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50436-9990-1 120 g in 1 BOTTLE; Type 0: Not a Combination Product 01/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/15/2011 Labeler - Unit Dose Services (831995316) Establishment Name Address ID/FEI Business Operations Unit Dose Services 831995316 REPACK(50436-9990) , RELABEL(50436-9990)