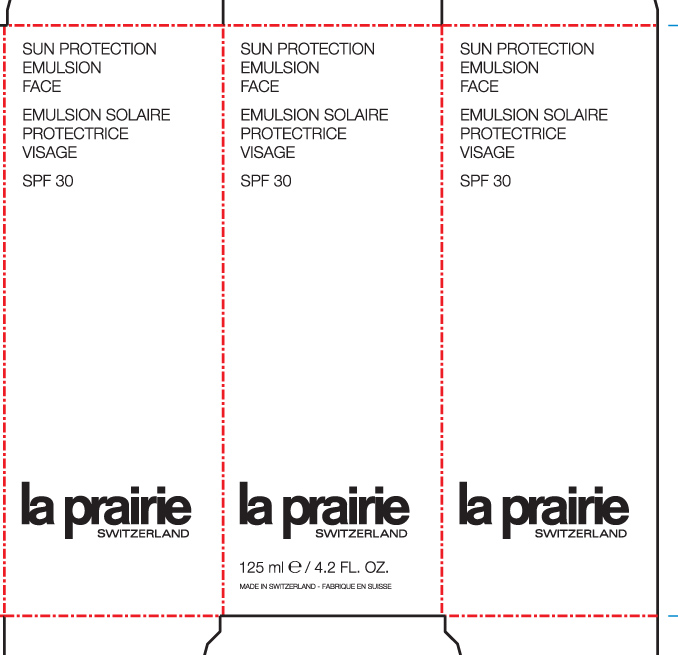
Label: SUN PROTECTION EMULSION FACE- avobenzone, homosalate, octisalate, octocrylene, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 59614-221-01, 59614-221-02, 59614-221-11 - Packager: Juvena GMBH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 14, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients Purpose
Avobenzone 3.0% sunscreen
Homosalate 10.0% sunscreen
Octisalate 2.6% sunscreen
octocrylene 2.4% sunscreen
Oxybenzone 5.0% sunscreen
- Helps prevent sunburn
- for skin that burns easily
-provides moderate protection against sunburn
-higher SPF gives more sunburn protection
Apply daily after cleansing and toning .
Smooth over face and throat.
Apply evenly before sun exposure.
Reapply as needed or after towel drying, swimming or perspiring.
Water (Aqua), Caprylyl Methicone, Hexyldecanol, Dimethicone, Polyglyceryl-3 Methylglucose Distearate, Undercrylene Dimethicone, Aluminum Starch Octenylsuccinate, Steareth-21, Octyldodecyl Olivate, Cetyl Alcohol, Silica, Glycerin, Glycoproteins, Panax Ginseng Root Extract*, Equisetum Arvense (Horsetail) Extract*, Sodium Hyaluronate, Corallina Officinalis Extract, Sea Water ( Maris Aqua), Algae Extract, Aloe Barbadensis Leaf Juice, Steareth-2, Ethylhexyl Glycerin, Disodium EDTA, Benzylidene Dimethoxydimethylindanone, sodium hydroxide, porphyra Umbilicalis Extract, Squalane, Carbomer, Potassium Sorbate, Hydroxyethyl acrylate/ Sodium Acryloyldimethyl Taurate Copolymer, Palmaria Pakmata Extract, Tocopheryl Acetate, Diethylhexyl Syringyldenemalonate, Pelvetia Canaliculata Extract, Polysorbate 60, Lecithin, Caprylic/ Capric Triglyceride, Sodium Lactate, Caprylyl Glycol, Alcohol, Benzyl Alcohol, Fragrance ( parfum), Phenoxyethanol, Chlorphenesin, Sodium Benzoate, Methylparaben, Ethylparaben, Propyparaben

- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUN PROTECTION EMULSION FACE LA PRAIRIE
avobenzone, homosalate, octisalate, octocrylene, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59614-221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 3 mL in 100 mL Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 10 mL in 100 mL Octisalate (UNII: 4X49Y0596W) (octisalate - UNII:4X49Y0596W) Octisalate 2.5 mL in 100 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 2.4 mL in 100 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 5 mL in 100 mL Inactive Ingredients Ingredient Name Strength Hexyldecanol (UNII: 151Z7P1317) Dimethicone (UNII: 92RU3N3Y1O) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) STEARETH-21 (UNII: 53J3F32P58) CETYL ALCOHOL (UNII: 936JST6JCN) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) STEARETH-2 (UNII: V56DFE46J5) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) squalane (UNII: GW89575KF9) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYSORBATE 60 (UNII: CAL22UVI4M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SODIUM LACTATE (UNII: TU7HW0W0QT) CAPRYLYL GLYCOL (UNII: 00YIU5438U) alcohol (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) SODIUM BENZOATE (UNII: OJ245FE5EU) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59614-221-11 0.088 mL in 1 DRUM 2 NDC:59614-221-02 1 in 1 BOX 2 NDC:59614-221-01 125 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 02/14/2011 Labeler - Juvena GMBH (315053785) Registrant - Juvena GMBH (315053785) Establishment Name Address ID/FEI Business Operations Temmentec Ag 480586411 manufacture