Label: NITROGEN gas
-
NDC Code(s):
48546-0003-1,
48546-0003-2,
48546-0003-3,
48546-0003-4, view more48546-0003-5, 48546-0003-6, 48546-0003-7, 48546-0003-8, 48546-0003-9, 48546-0005-1, 48546-0005-2, 48546-0005-3, 48546-0005-4
- Packager: Indiana Oxygen Company Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 24, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
NITROGEN COMPRESSED LABEL
NITROGEN, COMPRESSED NF UN1066
NON-FLAMMABLE GAS 2 35149 (01/15)
Rx only. WARNING: Administration of Nitrogen may be hazardous or contraindicated. For use only by or under the supervision of a licensed practioner who is experienced in the use and administration of Nitrogen and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications and side effects and the precautions to be taken. WARNING: CONTAINS GAS UNDER PRESSURE; MAY EXPLODE, IF HEATED, MAY DISPLACE OXYGEN AND CAUSE RAPID SUFFOCCATION. Do not handle until all safety precautions have been read and understood. Use and store only outdoors or in a well-ventilated place. Use a back flow preventive device in the piping. Use only with equipment rated for cylinder pressure. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use. FIRST AID; IF INHALED; Remove person to fresh air and keep comfortable for breathing. Get medical advice/attention. CAS: 7727-37-9 DO NOT REMOVE THIS PRODUCT LABEL
INDIANA OXYGEN COMPANY
6099 WEST CORPORATE WAY • INDIANAPOLIS, IN 46278
317-290-0003


-
NITROGEN REFRIGERATED LIQUID LABEL
REFRIGERATED LIQUID
NITROGEN, REFRIGERATED LIQUID NF UN1977
NON-FLAMMABLE GAS 2 (R-K14) 115149
↑ALWAYS KEEP CONTAINER IN UPRIGHT POSITION.↑
DO NOT CHANGE OR FORCE FIT CONNECTIONS.
RX ONLY
CONTENTS: ___________________________________ LTRS: ___ CU FT: ___
WARNING: Administration of Nitrogen may be hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use and administration of Nitrogen and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications and side effects and the precautions to be taken.
WARNING: CONTAINS REFRIGERATED GAS; MAY CAUSE CRYOGENIC BURNS OR INJURY. MAY DISPLACE OXYGEN AND CAUSE RAPID SUFFOCATION.
Do not handle until all safety precautions have been read and understood.
Use and store only outdoors or in a well-ventilated place. Wear cold insulating gloves, face shield, and eye protection. Use a back-flow preventive device in the piping. DO NOT change or force fit connections.
Close valve after each use and when empty.
Always keep container in upright position.
Read and follow the Safety Data Sheet (SDS) before use.
FIRST AID: IF INHALED: Remove person to fresh air and keep comfortable for breathing.
IF ON SKING: Thaw frosted parts with lukewarm water. Do not rub affected area. Get immediate medical advice/attention.
CAS: 7727-37-9
DO NOT REMOVE THIS PRODUCT LABEL.
INDIANA OXYGEN COMPANY
6099 West Corporate Way
Indianapolis, IN 46278
317-290-0003


-
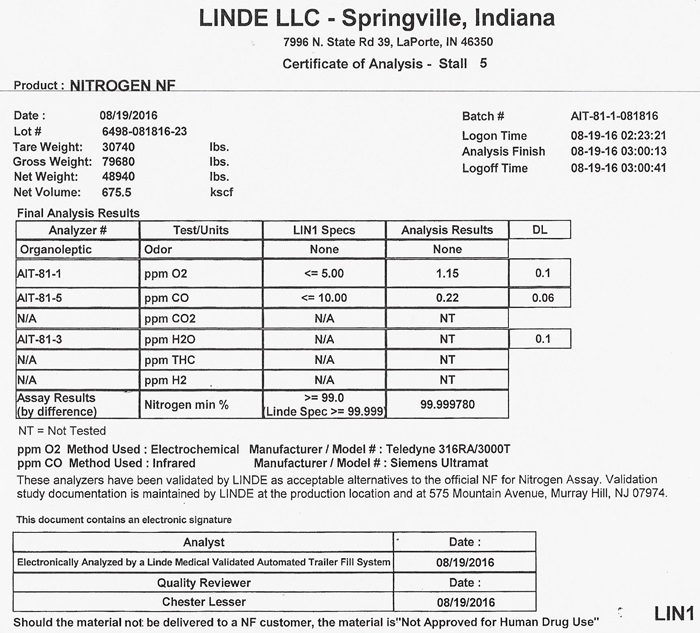
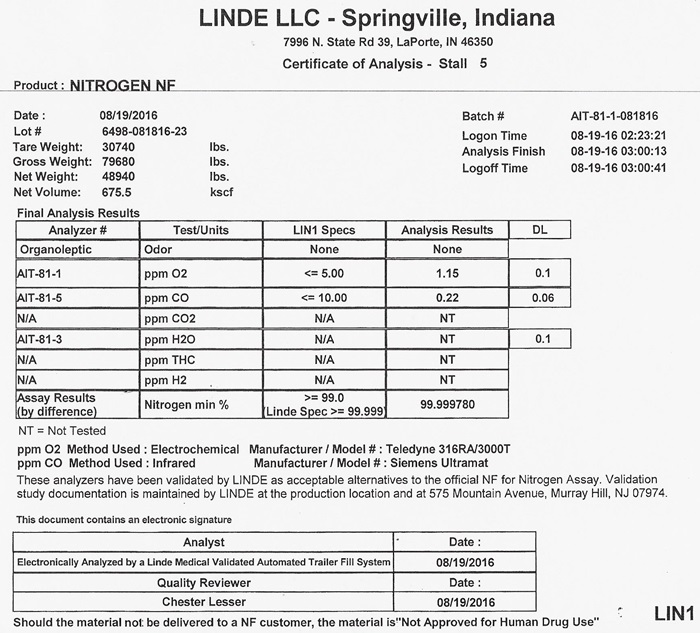
NITROGEN CERTIFICATE OF ANALYSIS
Certificate of Analysis - Nitrogen NF Customer ________________________ Vendor / Supplier _____________________________ This letter is to provide you with the certification you requested for Nitrogen NF, Lot number ___________. supplied to you in cylinders / vessels by our location. These cylinders were filled in accordance with the standard operating procedures utilized for the manufacture of Medical Gases. By following these procedures, our location ensures that products are safely manufactured in compliance with FDA's Current Good Manufacturing Regulations. CAUTION: Vendor supplies this certification to customer to assist customer in ensuring compliance with 21 CFR 211.84. This certification does not eliminate customer's obligation to cmply with other portions of 21 CFR 210 and 211 including but not limited to 21 CFR 211.165 (finished product testing) for cylinders and vessels filled from these supply cylinders. These cylinders are not certified for instrument calibration purposes.
TEST/REQUIREMENT NF SPECIFICATION LOT ANALYSIS
IDENTIFICATION PASS
ODOR NO APPRECIABLE ODOR
OXYGEN LESS THAN 1.0%
CARBON MONOXIDE LESS THAN 10 PPM
ASSAY GREATER THAN 99.0%THE METHODOLOGY BEING USED TO PERFORM THE NF TEST FOR ASSAY IS INDICATED BELOW:
________________________ PARAMAGNETIC ANALYZER MODEL NUMBER ___________________
________________________ ELECTROCHEMICAL ANALYZER MODEL NUMBER ______________
_______________________ GAS CHROMATOGRAPHY OFFICIAL USP / NF METHOD
SUPPLIER SIGNATURE ___________________ DATE _________________
J 700 a1 (01/03) BR COMPLIANCE ASSOCIATES LLC

-
INGREDIENTS AND APPEARANCE
NITROGEN
nitrogen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:48546-0003 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGEN (UNII: N762921K75) (NITROGEN - UNII:N762921K75) NITROGEN 99 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48546-0003-1 6400 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1975 2 NDC:48546-0003-2 4220 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/1975 3 NDC:48546-0003-3 1140 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1975 4 NDC:48546-0003-4 2280 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1975 5 NDC:48546-0003-5 3190 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1975 6 NDC:48546-0003-6 4890 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1975 7 NDC:48546-0003-7 6340 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1975 8 NDC:48546-0003-8 8420 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1975 9 NDC:48546-0003-9 119935 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/1975 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205766 01/01/1975 NITROGEN
nitrogen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:48546-0005 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGEN (UNII: N762921K75) (NITROGEN - UNII:N762921K75) NITROGEN 99.1 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48546-0005-1 136321 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/1975 2 NDC:48546-0005-2 152339 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/1975 3 NDC:48546-0005-3 152339 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/1975 4 NDC:48546-0005-4 253908 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/1975 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205766 01/01/1975 Labeler - Indiana Oxygen Company Inc (006036776) Registrant - Indiana Oxygen Company Inc (006036776) Establishment Name Address ID/FEI Business Operations Indiana Oxygen Company Inc 006036776 manufacture(48546-0003, 48546-0005)