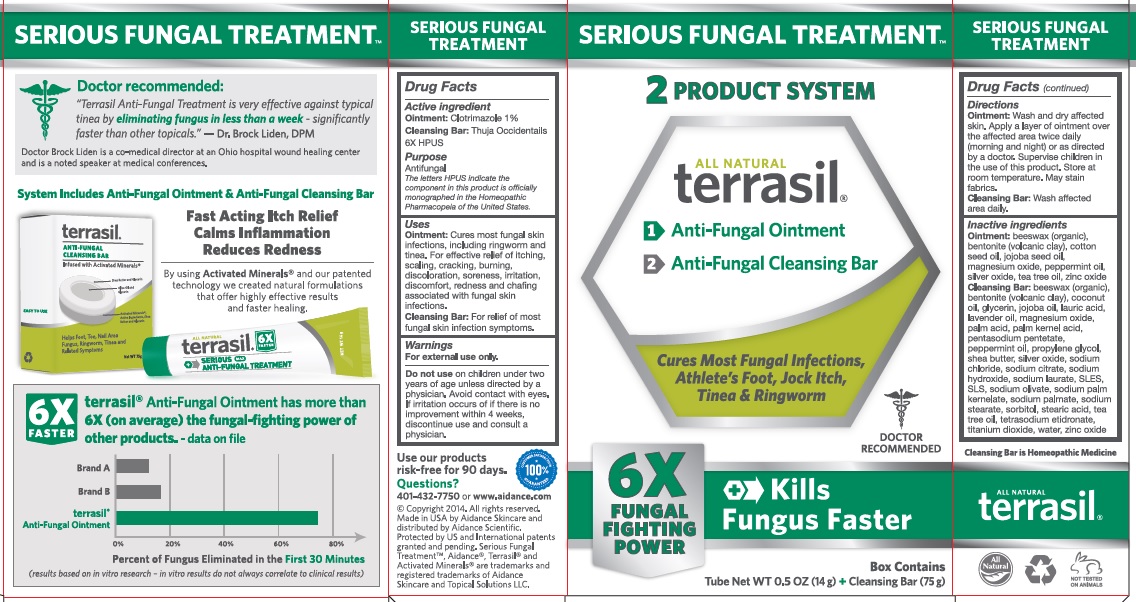
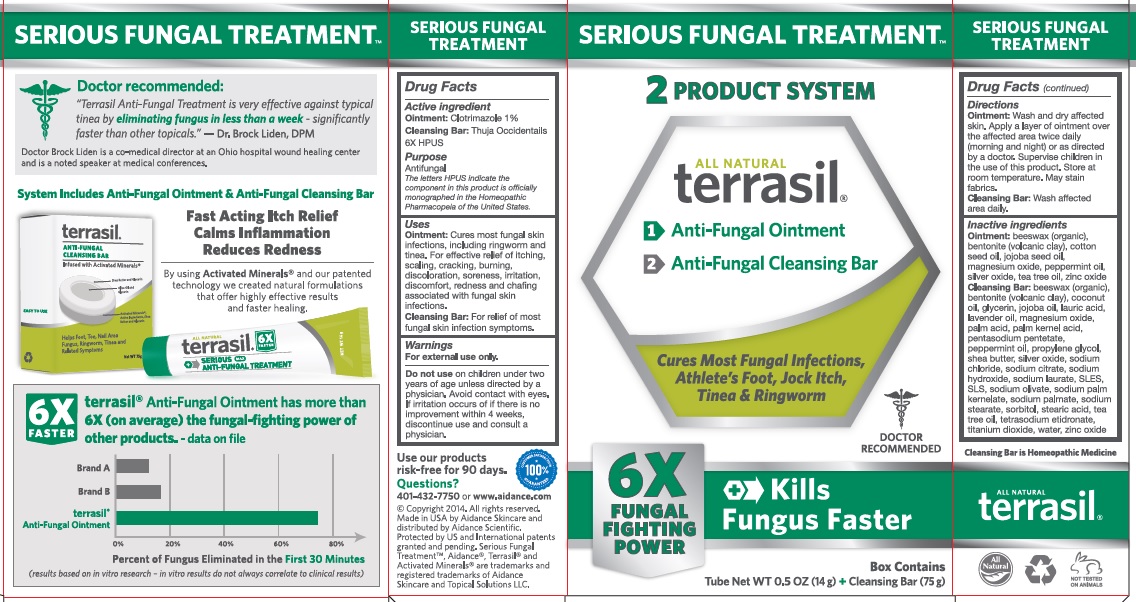
Label: TERRASIL SERIOUS FUNGAL TREATMENT- clotrimazole, thuja occidentalis kit
- NDC Code(s): 24909-155-25, 24909-156-14, 24909-157-75
- Packager: Aidance Skincare & Topical Solutions, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
Uses
Ointment: Cures most fungal skin infections, including ringworm and tinea. For effective relief of itching, scaling, cracking, burning, discoloration, soreness, irritation, discomfort, redness and chafing associated with fungal skin infections.
Cleansing Bar: For relief of most fungal skin infection symptoms.
- Warnings
- Directions
-
Inactive Ingredients
Ointment:beeswax (organic), bentonite (volcanic clay), cotton seed oil, jojoba seed oil, magnesium oxide, peppermint oil, silver oxide, tea tree oil, zinc oxide.
Cleansing Bar: beeswax (organic), bentonite (volcanic clay), coconut oil, glycerin, jojoba seed oil, lauric acid, lavender oil, magnesium oxide, palm acid, palm kernel acid, pentasodium pentetate, peppermint oil, propylene glycol, shea butter, silver oxide, sodium chloride, sodium citrate, sodium hydroxide, sodium laurate, SLES, SLS, sodium olvate, sodium palm kernelate, sodium palmate, sodium stearate, sorbitol, stearic acid, tea tree oil, tetrasodium etidronate, titanium dioxide, water, zinc oxide.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TERRASIL SERIOUS FUNGAL TREATMENT
clotrimazole, thuja occidentalis kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24909-155 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24909-155-25 1 in 1 PACKAGE; Type 1: Convenience Kit of Co-Package 01/01/2017 09/22/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 14 g Part 2 1 CARTON 75 g Part 1 of 2 TERRASIL SERIOUS ANTI-FUNGAL
clotrimazole ointmentProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 1 g in 100 g Inactive Ingredients Ingredient Name Strength BENTONITE (UNII: A3N5ZCN45C) COTTONSEED OIL (UNII: H3E878020N) JOJOBA OIL (UNII: 724GKU717M) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) PEPPERMINT OIL (UNII: AV092KU4JH) SILVER OXIDE (UNII: 897WUN6G6T) TEA TREE OIL (UNII: VIF565UC2G) WHITE WAX (UNII: 7G1J5DA97F) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24909-156-14 14 g in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 01/01/2017 09/22/2024 Part 2 of 2 TERRASIL ANTI-FUNGAL CLEANSING BAR
thuja occidentalis soapProduct Information Item Code (Source) NDC:24909-157 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THUJA OCCIDENTALIS WHOLE (UNII: 5HBV6WCE3N) (THUJA OCCIDENTALIS WHOLE - UNII:5HBV6WCE3N) THUJA OCCIDENTALIS WHOLE 6 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength BENTONITE (UNII: A3N5ZCN45C) COCONUT OIL (UNII: Q9L0O73W7L) GLYCERIN (UNII: PDC6A3C0OX) JOJOBA OIL (UNII: 724GKU717M) LAURIC ACID (UNII: 1160N9NU9U) LAVENDER OIL (UNII: ZBP1YXW0H8) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) PALM ACID (UNII: B6G0Y5Z616) PALM KERNEL ACID (UNII: 79P21R4317) PENTASODIUM PENTETATE (UNII: 961TOZ5L7T) PEPPERMINT OIL (UNII: AV092KU4JH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) SILVER OXIDE (UNII: 897WUN6G6T) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAURATE (UNII: K146MR5EXO) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) SODIUM PALM KERNELATE (UNII: 6H91L1NXTW) SODIUM PALMATE (UNII: S0A6004K3Z) SODIUM STEARATE (UNII: QU7E2XA9TG) SORBITOL (UNII: 506T60A25R) STEARIC ACID (UNII: 4ELV7Z65AP) TEA TREE OIL (UNII: VIF565UC2G) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ETIDRONATE TETRASODIUM (UNII: CZZ9T1T1X4) WATER (UNII: 059QF0KO0R) WHITE WAX (UNII: 7G1J5DA97F) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24909-157-75 75 g in 1 CARTON; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2017 09/22/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 01/01/2017 Labeler - Aidance Skincare & Topical Solutions, LLC (018950611) Establishment Name Address ID/FEI Business Operations Aidance Skincare & Topical Solutions, LLC 018950611 manufacture(24909-155) , label(24909-155)