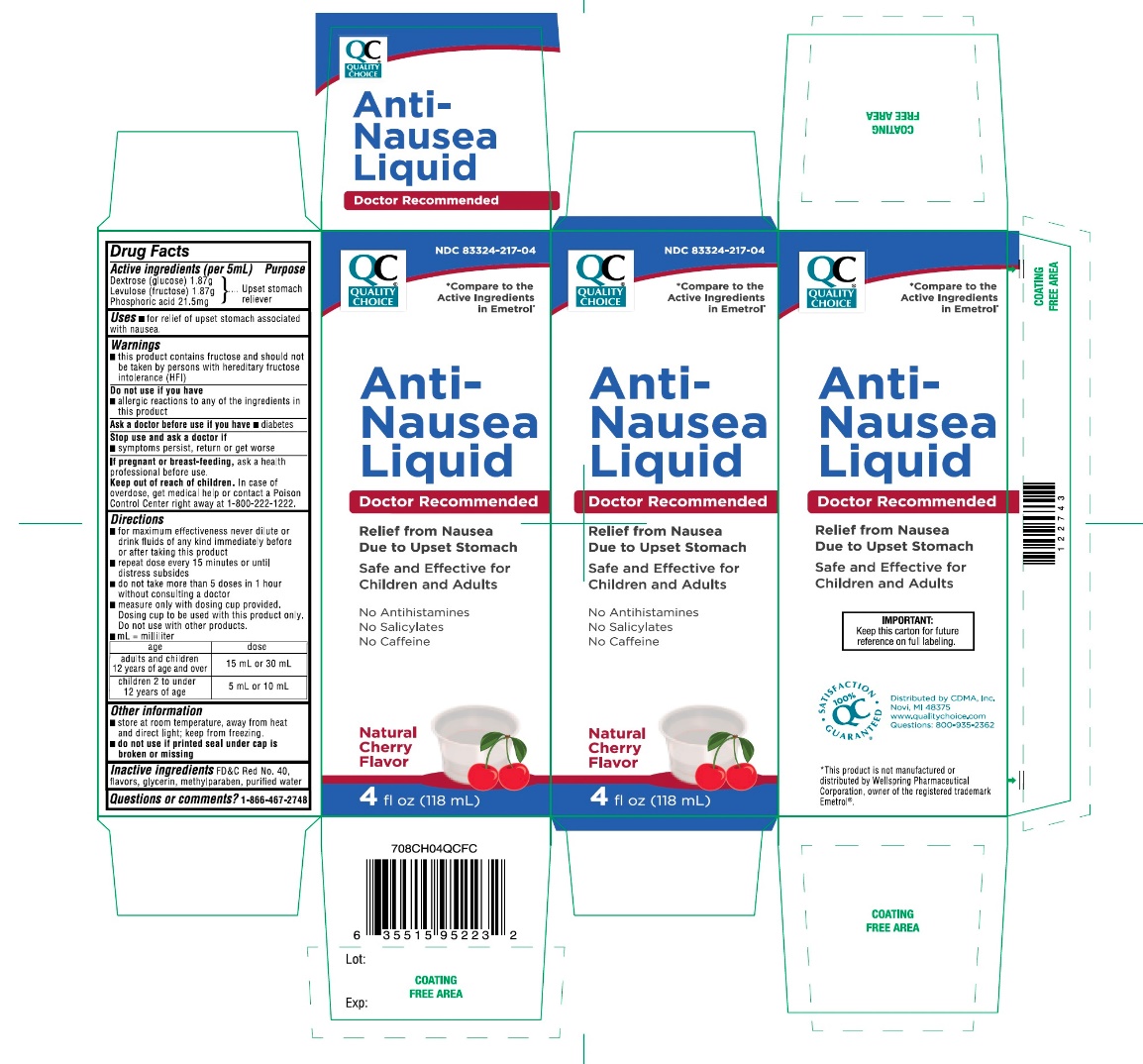
Label: QUALITY CHOICE ANTI-NAUSEA (dextrose(glucose),levulose- fructose,phosphoric acid solution
- NDC Code(s): 83324-217-04
- Packager: QUALITY CHOICE (CHAIN DRUG MARKETING ASSOCIATION)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (per 5 mL)
- Purpose
- Uses
- Warnings
-
Directions
- ▪
- for maximum effectiveness never dilute or drink fluids of any kind immediately before or after taking this product
- ▪
- repeat dose every 15 minutes or until distress subsides
- ▪
- do not take more than 5 doses in 1 hour without consulting a doctor
- ▪
- measure only with dosing cup provided. Dosing cup to be used with this product only. Do not use with other products.
- ▪
- mL= milliliter
- age
- dose
- adults and children 12 years of age and over
- 15 mL or 30 mL
- children 2 to under 12 years of age
- 5 ml or 10 mL
- Other information
- Inactive ingredients
- Questions or Comments?
-
SPL UNCLASSIFIED SECTION
*Compare to the Active Ingredients Emetrol®
NDC 83324-217-04
Anti-Nausea Liquid
Doctor Recommended
Relief from Nausea Due to Upset Stomach Safe and Effective for Children and Adults
- No Antihistamines
- No Salicylates
- No Caffeine
Natural Cherry Flavor
4 fl oz (118 mL)
100% QC SATISFACTION GUARANTEED
IMPORTANT: Keep this carton for future reference on full labeling.
Distributed by CDMA, Inc.
Novi, MI 48375
Questions: 800-935-2362
*This product is not manufactured or distributed by Wellspring Pharmaceutical Corporation., owner of the registered trademark Emetrol®.
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
QUALITY CHOICE ANTI-NAUSEA
dextrose(glucose),levulose(fructose),phosphoric acid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83324-217 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE, UNSPECIFIED FORM 1.87 g in 5 mL FRUCTOSE (UNII: 6YSS42VSEV) (FRUCTOSE - UNII:6YSS42VSEV) FRUCTOSE 1.87 g in 5 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 21.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) Product Characteristics Color RED (Red) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83324-217-04 1 in 1 CARTON 10/08/2024 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 04/16/2019 Labeler - QUALITY CHOICE (CHAIN DRUG MARKETING ASSOCIATION) (011920774)