Label: AGE DEFYING BLEMISH TREATMENT- salicylic acid cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 44717-148-01, 44717-148-02 - Packager: Wasatch Product Development, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 12, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredient Purpose
Salicylic Acid 2% Acne Treatment
Uses
For the treatment of acne
Dries up acne pimples
Helps prevent new acne pimples
Keep out of reach of children. If swallowed, get medical help or contact a a Poison Control Center right away.
Stop use and ask a doctor if irritation becomes severe.
Warnings
For external use only
When using this product skin irritation and dryness is more likely to occur if you use another topical acne medication. If irritation occurs, start with one application daily, then gradually increase to two or three times daily if needed or directed by a doctor.
If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Directions
Clean the skin thoroughly before applying this product
Cover the entire affected area with a thin layer one to three times daily
Becuase excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three daily if needed or directed by a doctor.
Inactive ingredients SD Alcohol 40-B, Ethoxydiglycol, Water, Glycerin, Azelaic Acid, Nylon-10/10, Polyacrylate Crosspolymer-6, Propanediol, Betaine, PEG-8/SMDI Copolymer, PEG-12 Dimethicone, Palmitoyl Tripeptide-38, Lens Esculenta (Lentil) Seed Extract, Tetrahexyldecyl Ascorbate, Dipotassium Glycyrrhizinate, Hydroxypropyl Cyclodextrin, Triethanolamine, Fragrance
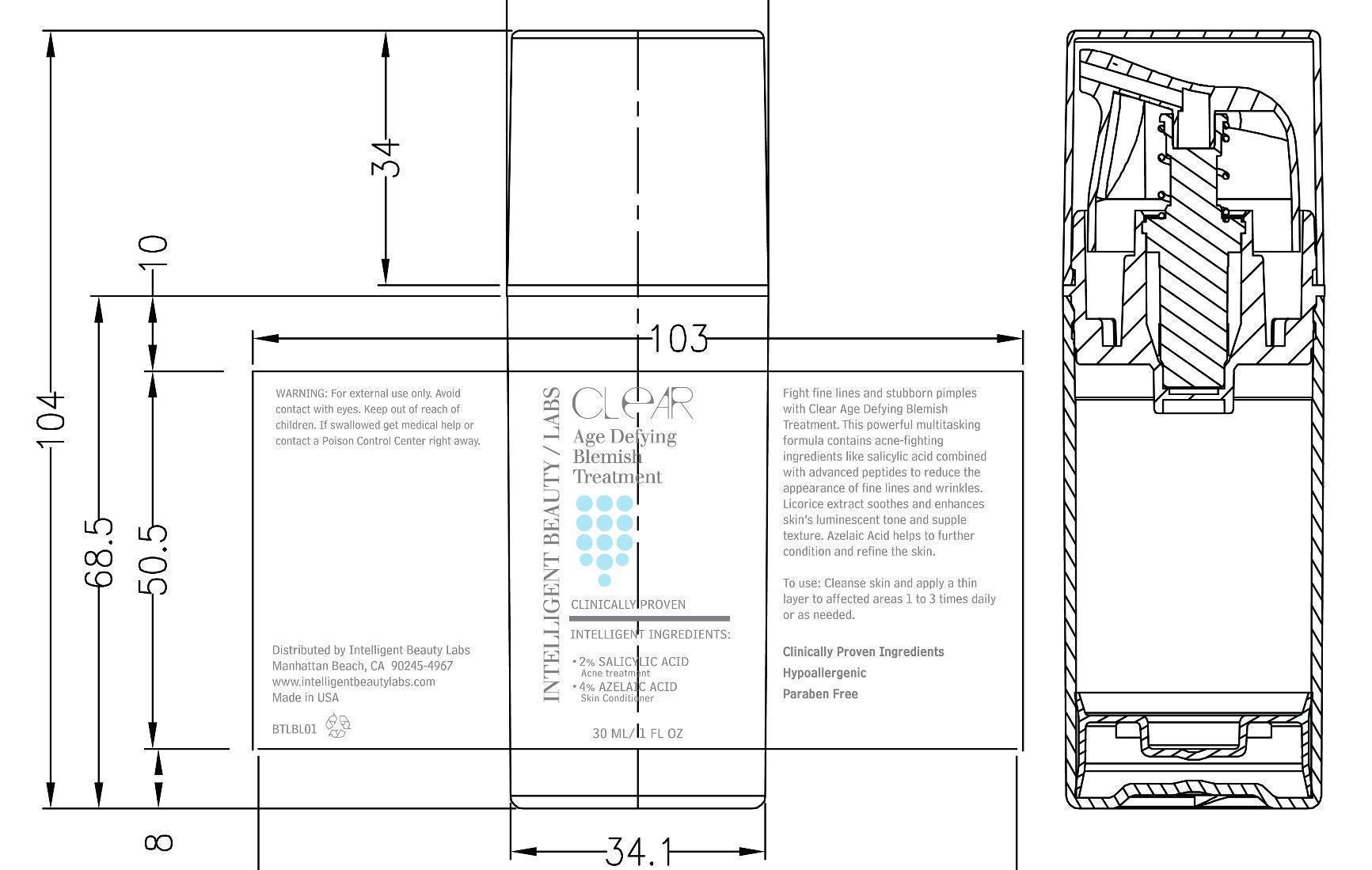
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AGE DEFYING BLEMISH TREATMENT
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44717-148 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) AZELAIC ACID (UNII: F2VW3D43YT) PROPANEDIOL (UNII: 5965N8W85T) BETAINE (UNII: 3SCV180C9W) PEG-8/SMDI COPOLYMER (UNII: CCX72L6NY6) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) LENTIL (UNII: 6O38V6B52O) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) HYDROXYPROPYL .ALPHA.-CYCLODEXTRIN (UNII: ZFR0T80O4Y) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44717-148-02 1 in 1 CARTON 1 NDC:44717-148-01 30 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 10/24/2013 Labeler - Wasatch Product Development, LLC (962452533) Registrant - Wasatch Product Development, LLC (962452533) Establishment Name Address ID/FEI Business Operations Wasatch Product Development, LLC 962452533 manufacture(44717-148)