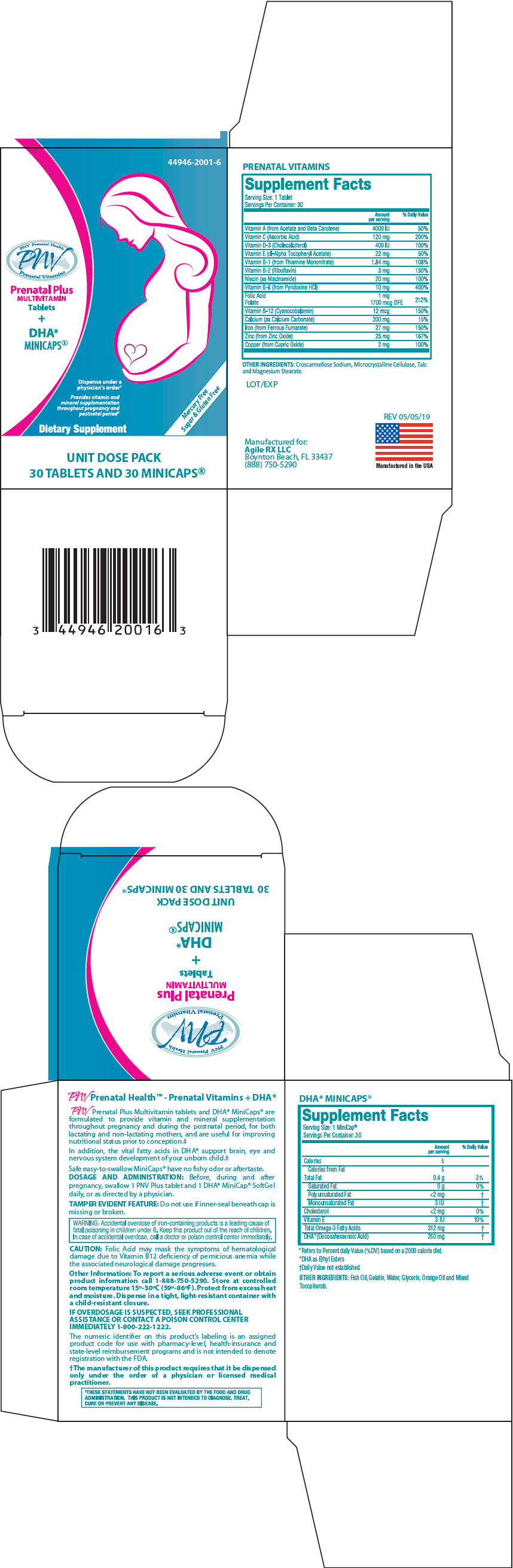
Label: PRENATAL PLUS MULTIVITAMIN PLUS DHA MINICAPS- vitamin a, ascorbic acid, cholecalciferol, tocopherol, thiamine mononitrate, riboflavin, pyridoxine, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, zinc oxide, cupric oxide, niacinamide, and fish oil kit
- NHRIC Code(s): 44946-2001-6
- Packager: Agile RX
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated November 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
PRENATAL VITAMINS Supplement Facts Serving Size: 1 Tablet Servings Per Container: 30 Amount per serving % Daily Value OTHER INGREDIENTS: Croscarmellose Sodium, Microcrystalline Cellulose, Talc and Magnesium Stearate. Vitamin A (from Acetate and Beta Carotene) 4000 IU 50% Vitamin C (Ascorbic Acid) 120 mg 200% Vitamin D-3 (Cholecalciferol) 400 IU 100% Vitamin E (dl-Alpha Tocopheryl Acetate) 22 mg 50% Vitamin B-1 (from Thiamine Mononitrate) 1.84 mg 108% Vitamin B-2 (Riboflavin) 3 mg 150% Niacin (as Niacinamide) 20 mg 100% Vitamin B-6 (from Pyridoxine HCl) 10 mg 400% Folic Acid
Folate1 mg
1700 mcg DFE212% Vitamin B-12 (Cyanocobalamin) 12 mcg 150% Calcium (as Calcium Carbonate) 200 mg 15% Iron (from Ferrous Fumarate) 27 mg 150% Zinc (from Zinc Oxide) 25 mg 167% Copper (from Cupric Oxide) 2 mg 100% DHA* MINICAPS® Supplement Facts Serving Size: 1 MiniCap® Servings Per Container: 30 Amount per serving % Daily Value *Refers to Percent daily Value (%DV) based on a 2000 calorie diet. *DHA as Ethyl Esters †Daily Value not established. OTHER INGREDIENTS: Fish Oil, Gelatin, Water, Glycerin, Orange Oil and Mixed Tocopherols. Calories 5 Calories from Fat 5 Total Fat 0.4 g 3% Saturated Fat 0 g 0% Polyunsaturated Fat <2 mg † Monounsaturated Fat 3 IU † Cholesterol <2 mg 0% Vitamin E 3 IU 10% Total Omega-3 Fatty Acids 312 mg † DHA*(Docosahexaenoic Acid) 250 mg † -
HEALTH CLAIM
PNV Prenatal Plus Multivitamin tablets and DHA* MiniCaps® are formulated to provide vitamin and mineral supplementation throughout pregnancy and during the postnatal period, for both lactating and non-lactating mothers, and are useful for improving nutritional status prior to conception.‡
In addition, the vital fatty acids in DHA* support brain, eye and nervous system development of your unborn child.‡
Safe easy-to-swallow MiniCaps® have no fishy odor or aftertaste.
- DOSAGE AND ADMINISTRATION
- TAMPER EVIDENT FEATURE
- WARNINGS
- CAUTION
- Other Information
- HEALTH CLAIM
-
HEALTH CLAIM
The numeric identifier on this product's labeling is an assigned product code for use with pharmacy-level, health-insurance and state level reimbursement programs and is not intended to denote registration with the FDA.
†The manufacturer of this product requires that it be dispensed only under the order of a physician or licensed medical practitioner.
‡THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE. - HEALTH CLAIM
-
PRINCIPAL DISPLAY PANEL - Kit Carton
44946-2001-6
PNV Prenatal Health
PNV
Prenatal VitaminsPrenatal Plus
MULTIVITAMIN
Tablets
+
DHA*
MINICAPS®Dispense under a
physician's order†Provides vitamin and
mineral supplementation
throughout pregnancy and
postnatal period‡Dietary Supplement
Mercury Free
Sugar & Gluten FreeUNIT DOSE PACK
30 TABLETS AND 30 MINICAPS®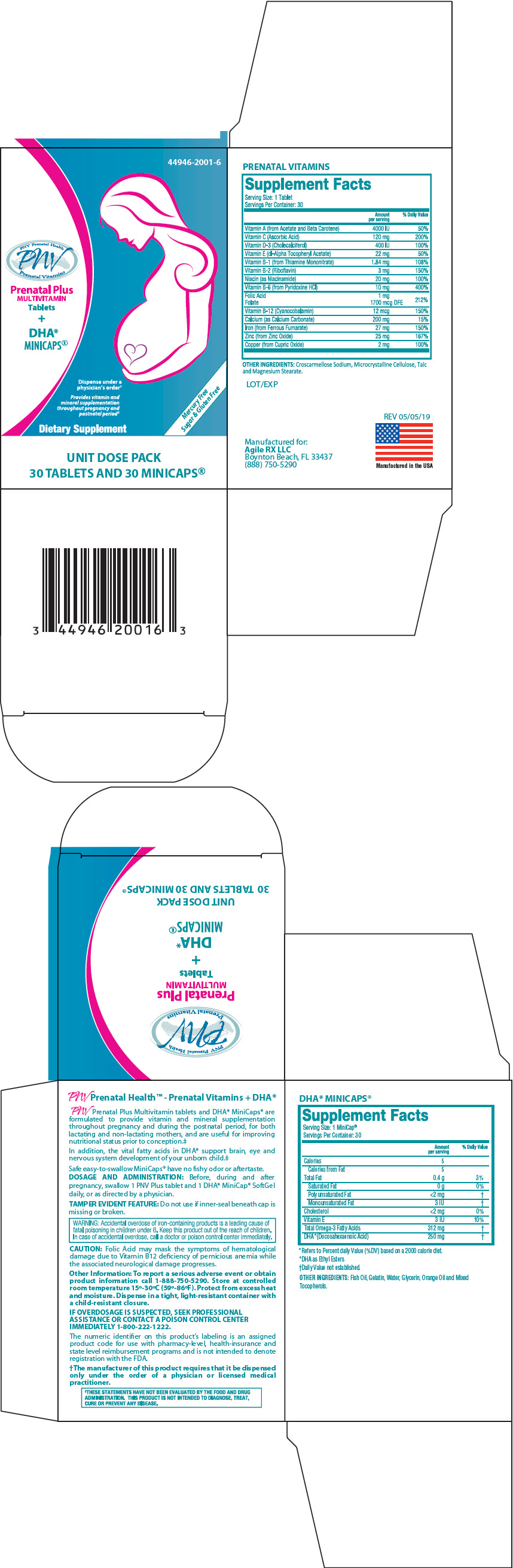
-
INGREDIENTS AND APPEARANCE
PRENATAL PLUS MULTIVITAMIN PLUS DHA MINICAPS
vitamin a, ascorbic acid, cholecalciferol, tocopherol, thiamine mononitrate, riboflavin, pyridoxine, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, zinc oxide, cupric oxide, niacinamide, and fish oil kitProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:44946-2001 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:44946-2001-6 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 30 Part 2 1 BLISTER PACK 30 Part 1 of 2 PRENATAL PLUS MULTIVITAMIN
vitamin a, ascorbic acid, cholecalciferol, tocopherol, thiamine mononitrate, riboflavin, pyridoxine, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, zinc oxide, cupric oxide, and niacinamide tabletProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 2000 [iU] .BETA.-CAROTENE (UNII: 01YAE03M7J) (.BETA.-CAROTENE - UNII:01YAE03M7J) .BETA.-CAROTENE 2000 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 22 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.84 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 10 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 200 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 27 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 25 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE (UNII: 029TFK992N) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 11/15/2016 Part 2 of 2 DHA MINICAPS
fish oil capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FISH OIL (UNII: XGF7L72M0F) (FISH OIL - UNII:XGF7L72M0F) FISH OIL 312 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ORANGE OIL (UNII: AKN3KSD11B) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 11/15/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 11/15/2016 Labeler - Agile RX (116742427)