Label: ALLERGY- chlorpheniramine maleate tablet
- NDC Code(s): 0904-0012-24, 0904-0012-59, 0904-0012-61, 0904-0012-80
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
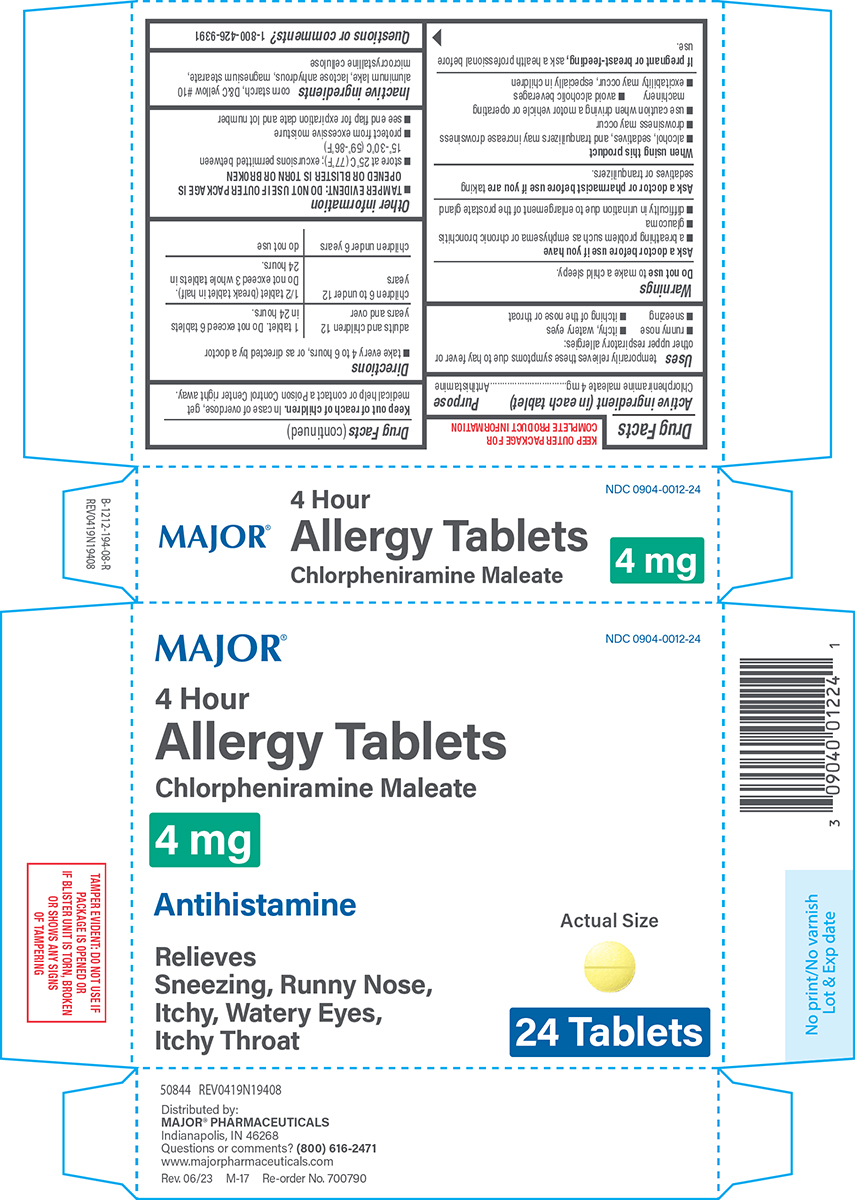
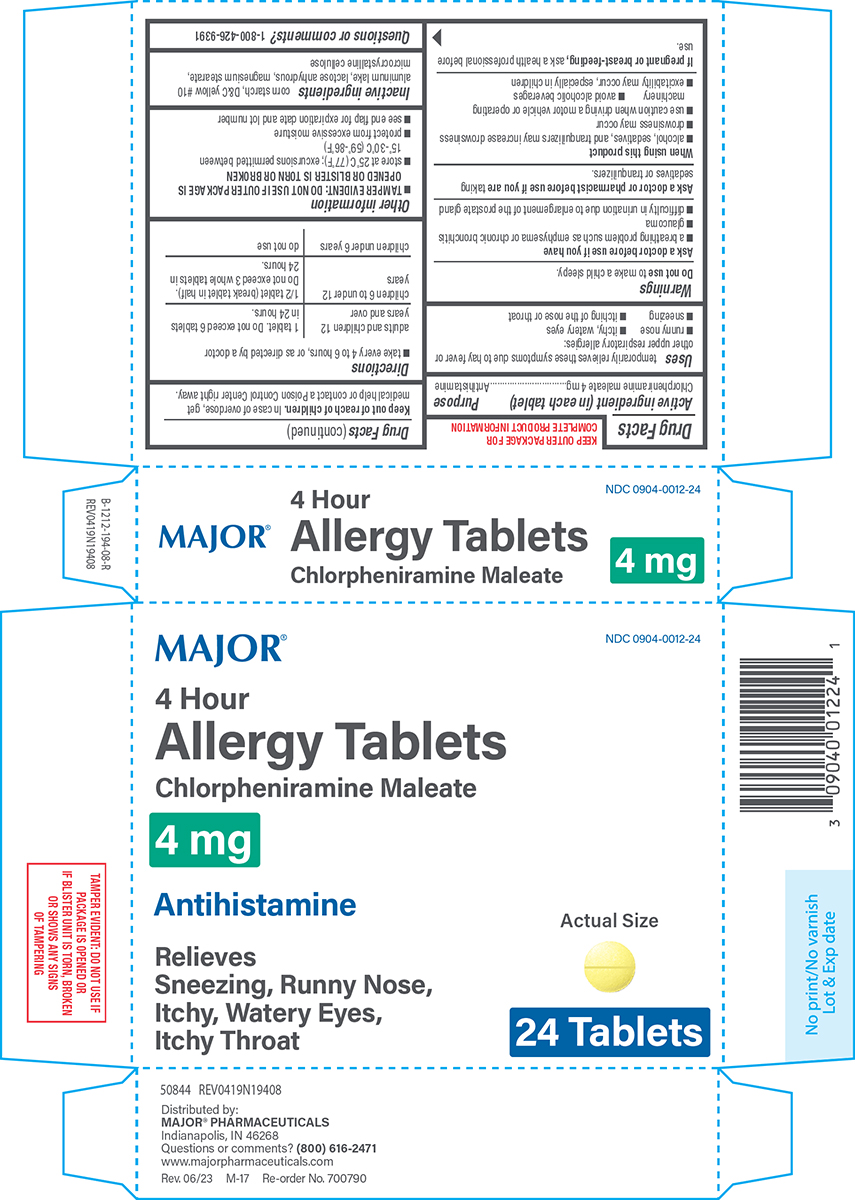
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
NDC 0904-0012-24
MAJOR®
4 Hour
Allergy Tablets
Chlorpheniramine Maleate
4 mg
AntihistamineRelieves
Sneezing, Runny Nose,
Itchy, Watery Eyes,
Itchy ThroatActual Size
24 Tablets
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR
IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS
OF TAMPERING50844 REV0419N19408
Distributed by:
MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268
Questions or comments? (800) 616-2471
www.majorpharmaceuticals.comRev. 06/23 M-17 Re-order No. 700790
Major 44-194
-
Principal Display Panel - Unit Dose
NDC 0904-0012-61
MAJOR®
Unit Dose
ALLERGY TABLETS
CHLORPHENIRAMINE MALEATEAntihistamine
4 mg each
Institutional Dispensing Only
100 TABLETSTAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING50844 REV0419C19412
Distributed by:
MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268
(800) 616-2471
www.majorpharmaceuticals.comRev. 03/23 M-17 Re-order No. 301560

Major 44-194-DSP
-
INGREDIENTS AND APPEARANCE
ALLERGY
chlorpheniramine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-0012 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color yellow Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code 44;194 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-0012-24 2 in 1 CARTON 12/19/1992 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:0904-0012-59 1 in 1 CARTON 12/19/1992 2 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:0904-0012-80 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/19/1992 4 NDC:0904-0012-61 10 in 1 CARTON 12/19/1992 4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/19/1992 Labeler - Major Pharmaceuticals (191427277) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(0904-0012) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(0904-0012) , pack(0904-0012) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(0904-0012) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(0904-0012) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(0904-0012)