Label: CHLORDIAZEPOXIDE AND AMITRIPTYLINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 0378-0211-01, 0378-0211-05, 0378-0277-01, 0378-0277-05
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; DEPENDENCE AND WITHDRAWAL REACTIONS; and SUICIDAL THOUGHTS AND BEHAVIORS
- •
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGS and PRECAUTIONS).
- •
- The use of benzodiazepines, including chlordiazepoxide and amitriptyline hydrochloride tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing chlordiazepoxide and amitriptyline hydrochloride tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see WARNINGS).
- •
- The continued use of benzodiazepines, including chlordiazepoxide and amitriptyline hydrochloride tablets, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide and amitriptyline hydrochloride tablets after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide and amitriptyline hydrochloride tablets or reduce the dosage (see DOSAGE AND ADMINISTRATION and WARNINGS).
- •
- Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors (see WARNINGS). Chlordiazepoxide and amitriptyline hydrochloride tablets are not approved for use in pediatric patients (see PRECAUTIONS).
-
DESCRIPTION
Chlordiazepoxide and amitriptyline hydrochloride tablets, USP combine for oral administration, chlordiazepoxide, an agent for the relief of anxiety and tension, and amitriptyline, an antidepressant.
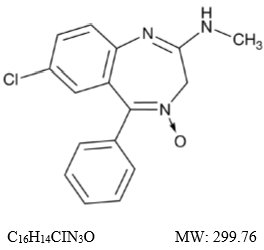
Chlordiazepoxide, USP is a benzodiazepine with the formula 7-chloro-2-(methylamino)-5-phenyl-3H-1,4-benzodiazepine-4-oxide. It is a yellow crystalline powder and is insoluble in water. The chemical structure is:
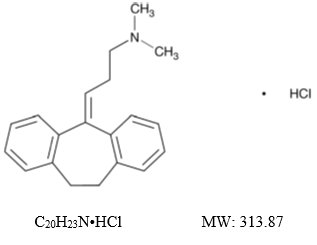
Amitriptyline hydrochloride, USP is a dibenzocycloheptadiene derivative. The formula is 10,11-dihydro-N,N-dimethyl-5H-dibenzo[a,d]cycloheptene-D5,g-propylamine hydrochloride. It is a white or practically white crystalline powder that is freely soluble in water. The chemical structure is:
Each film-coated tablet for oral administration contains 5 mg of chlordiazepoxide and 14 mg of amitriptyline hydrochloride equivalent to 12.5 mg of amitriptyline or 10 mg of chlordiazepoxide and 27.98 mg of amitriptyline hydrochloride equivalent to 25 mg of amitriptyline. Each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch (corn), sodium lauryl sulfate and titanium dioxide. In addition, the 5 mg/12.5 mg tablets also contain D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake and FD&C Yellow No. 6 Aluminum Lake and the 10 mg/25 mg tablets also contain polydextrose and triacetin.
-
CLINICAL PHARMACOLOGY
Both components of chlordiazepoxide and amitriptyline hydrochloride tablets exert their action in the central nervous system. Extensive studies with chlordiazepoxide in many animal species suggest action in the limbic system. Recent evidence indicates that the limbic system is involved in emotional response. Taming action was observed in some species. The mechanism of action of amitriptyline in man is not known, but the drug appears to interfere with the reuptake of norepinephrine into adrenergic nerve endings. This action may prolong the sympathetic activity of biogenic amines.
-
INDICATIONS AND USAGE
Chlordiazepoxide and amitriptyline hydrochloride tablets are indicated for the treatment of patients with moderate to severe depression associated with moderate to severe anxiety.
The therapeutic response to chlordiazepoxide and amitriptyline hydrochloride tablets occurs earlier and with fewer treatment failures than when either amitriptyline or chlordiazepoxide is used alone.
Symptoms likely to respond in the first week of treatment include: insomnia, feelings of guilt or worthlessness, agitation, psychic and somatic anxiety, suicidal ideation and anorexia.
-
CONTRAINDICATIONS
Chlordiazepoxide and amitriptyline hydrochloride tablets are contraindicated in patients with hypersensitivity to either benzodiazepines or tricyclic antidepressants. It should not be given concomitantly with a monoamine oxidase inhibitor. Hyperpyretic crises, severe convulsions and deaths have occurred in patients receiving a tricyclic antidepressant and a monoamine oxidase inhibitor simultaneously. When it is desired to replace a monoamine oxidase inhibitor with chlordiazepoxide and amitriptyline hydrochloride tablets, a minimum of 14 days should be allowed to elapse after the former is discontinued. Chlordiazepoxide and amitriptyline hydrochloride tablets should then be initiated cautiously with gradual increase in dosage until optimum response is achieved.
This drug is contraindicated during the acute recovery phase following myocardial infarction.
-
WARNINGS
Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including chlordiazepoxide and amitriptyline hydrochloride tablets, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe chlordiazepoxide and amitriptyline hydrochloride tablets concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of chlordiazepoxide and amitriptyline hydrochloride tablets than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking chlordiazepoxide and amitriptyline hydrochloride tablets, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when chlordiazepoxide and amitriptyline hydrochloride tablets are used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS: Drug-Drug Interactions).
Abuse, Misuse, and Addiction
The use of benzodiazepines, including chlordiazepoxide and amitriptyline hydrochloride tablets, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing chlordiazepoxide and amitriptyline hydrochloride tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of chlordiazepoxide and amitriptyline hydrochloride tablets, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of chlordiazepoxide and amitriptyline hydrochloride tablets along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide and amitriptyline hydrochloride tablets or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Chlordiazepoxide and Amitriptyline Hydrochloride Tablets).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including chlordiazepoxide and amitriptyline hydrochloride tablets, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide and amitriptyline hydrochloride tablets after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE: Dependence).
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE: Dependence).
Suicidal Thoughts and Behaviors in Adolescent and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1,000 patients treated are provided in Table 1.
Table 1: Risk Differences of the Number of Patients of Suicidal Thoughts and Behavior in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients Age Range
Drug-Placebo Difference in Number of Patients of Suicidal
Thoughts or Behaviors per 1,000 Patients TreatedIncreases Compared to Placebo
< 18 years old
14 additional patients
18 to 24 years
old5 additional patients
Decreases Compared to Placebo
25 to 64 years
old1 fewer patient
≥ 65 years old
6 fewer patients
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for any indication for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing chlordiazepoxide and amitriptyline hydrochloride tablets, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
Activation of Mania or Hypomania
In patients with bipolar disorder, treating a depressive episode with chlordiazepoxide and amitriptyline hydrochloride tablets or another antidepressant may precipitate a mixed/manic episode. Prior to initiating treatment with chlordiazepoxide and amitriptyline hydrochloride tablets, screen patients for any personal or family history of bipolar disorder, mania, or hypomania.
Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including chlordiazepoxide and amitriptyline hydrochloride tablets may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
General
Because of the atropine-like action of the amitriptyline component, great care should be used in treating patients with a history of urinary retention or angle-closure glaucoma. In patients with glaucoma, even average doses may precipitate an attack. Severe constipation may occur in patients taking tricyclic antidepressants in combination with anticholinergic-type drugs.
Patients with cardiovascular disorders should be watched closely. Tricyclic antidepressant drugs, particularly when given in high doses, have been reported to produce arrhythmias, sinus tachycardia and prolongation of conduction time. Myocardial infarction and stroke have been reported in patients receiving drugs of this class.
Because of the sedative effects of chlordiazepoxide and amitriptyline hydrochloride tablets, patients should be cautioned about combined effects with alcohol or other CNS depressants. The additive effects may produce a harmful level of sedation and CNS depression.
Patients receiving chlordiazepoxide and amitriptyline hydrochloride tablets should be cautioned against engaging in hazardous occupations requiring complete mental alertness, such as operating machinery or driving a motor vehicle.
Neonatal Sedation and Withdrawal Syndrome
Use of chlordiazepoxide and amitriptyline hydrochloride tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (see PRECAUTIONS: Pregnancy). Monitor neonates exposed to chlordiazepoxide and amitriptyline hydrochloride tablets during pregnancy or labor for signs of sedation and monitor neonates exposed to chlordiazepoxide and amitriptyline hydrochloride tablets during pregnancy for signs of withdrawal; manage these neonates accordingly.
-
PRECAUTIONS
General
Use with caution in patients with a history of seizures.
Close supervision is required when chlordiazepoxide and amitriptyline hydrochloride tablets are given to hyperthyroid patients or those on thyroid medication.
The usual precautions should be observed when treating patients with impaired renal or hepatic function.
Patients with suicidal ideation should not have easy access to large quantities of the drug. The possibility of suicide in depressed patients remains until significant remission occurs.
Information for Patients
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risks from Concomitant Use with Opioids
Advise both patients and caregivers about the risks of potentially fatal respiratory depression and sedation when chlordiazepoxide and amitriptyline tablets are used with opioids and not to use such drugs concomitantly unless supervised by a health care provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see WARNINGS: Risks from Concomitant Use with Opioids and PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction
Inform patients that the use of chlordiazepoxide and amitriptyline tablets, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see WARNINGS: Abuse, Misuse, and Addiction and DRUG ABUSE AND DEPENDENCE).
Withdrawal Reactions
Inform patients that the continued use of chlordiazepoxide and amitriptyline tablets may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of chlordiazepoxide and amitriptyline tablets may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of chlordiazepoxide and amitriptyline tablets may require a slow taper (see WARNINGS: Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidal thoughts and behaviors, especially early during treatment and when the dosage is adjusted up or down (see WARNINGS: Suicidal Thoughts and Behaviors in Adolescents and Young Adults).
Pregnancy
Advise pregnant females that use of chlordiazepoxide and amitriptyline hydrochloride tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome and Precautions, Pregnancy). Instruct patients to inform their healthcare provider if they are pregnant.
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to chlordiazepoxide and amitriptyline hydrochloride tablets during pregnancy (see PRECAUTIONS: Pregnancy).
Nursing
Advise patients that breastfeeding is not recommended during treatment with chlordiazepoxide and amitriptyline hydrochloride tablets (see PRECAUTIONS: Nursing Mothers).
Essential Laboratory Tests
Patients on prolonged treatment should have periodic liver function tests and blood counts.
Drug-Drug Interactions
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
Drug and Treatment Interactions
Because of its amitriptyline component, chlordiazepoxide and amitriptyline hydrochloride tablets may block the antihypertensive action of guanethidine or compounds with a similar mechanism of action.
Drugs Metabolized by P450 2D6
The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7% to 10% of Caucasians are so called “poor metabolizers”); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small or quite large (8-fold increase in plasma AUC of the TCA).
In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dose of TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the type 1c antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition. The extent to which SSRI TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the coadministration of TCAs with any of the SSRIs and also in switching from one class to the other. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary).
Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. Furthermore, whenever one of these other drugs is withdrawn from cotherapy, an increased dose of tricyclic antidepressant may be required. It is desirable to monitor TCA plasma levels whenever a TCA is going to be coadministered with another drug known to be an inhibitor of P450 2D6.
The effects of concomitant administration of chlordiazepoxide and amitriptyline hydrochloride tablets and other psychotropic drugs have not been evaluated. Sedative effects may be additive.
Cimetidine is reported to reduce hepatic metabolism of certain tricyclic antidepressants and benzodiazepines, thereby delaying elimination and increasing steady-state concentrations of these drugs. Clinically significant effects have been reported with the tricyclic antidepressants when used concomitantly with cimetidine (Tagamet®).
The drug should be discontinued several days before elective surgery.
Concurrent administration of ECT and chlordiazepoxide and amitriptyline hydrochloride tablets should be limited to those patients for whom it is essential.
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy registry that monitors pregnancy outcomes in women exposed to psychiatric medications, including chlordiazepoxide and amitriptyline hydrochloride tablets, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/pregnancyregistry/.
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome, and Clinical Considerations). Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia and sedation in neonates. Monitor neonates exposed to chlordiazepoxide and amitriptyline hydrochloride tablets during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to chlordiazepoxide and amitriptyline hydrochloride tablets during pregnancy for signs of withdrawal. Manage these neonates accordingly (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome).
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Nursing
Risk Summary
It is not known whether this drug is excreted in human milk. There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. Because of the potential for serious adverse reaction, including sedation and withdrawal symptoms in breastfed infants, advise patients that breastfeeding is not recommended during treatment with chlordiazepoxide and amitriptyline hydrochloride tablets.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established (see BOX WARNING and WARNINGS: Suicidal Thoughts and Behaviors in Adolescents and Young Adults).
Anyone considering the use of chlordiazepoxide and amitriptyline hydrochloride tablets in a child or adolescent must balance the potential risks with the clinical need.
Geriatric Use
In elderly and debilitated patients it is recommended that dosage be limited to the smallest effective amount to preclude the development of ataxia, oversedation, confusion or anticholinergic effects.
Of the total number of subjects in clinical studies of chlordiazepoxide and amitriptyline hydrochloride tablets, 74 individuals were 65 years and older. An additional 34 subjects were between 60 and 69 years of age. No overall differences in safety and effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
The active ingredients in chlordiazepoxide and amitriptyline hydrochloride tablets are known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of chlordiazepoxide and amitriptyline hydrochloride tablets and observed closely.
Clinical studies of chlordiazepoxide and amitriptyline hydrochloride tablets did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Adverse reactions to chlordiazepoxide and amitriptyline hydrochloride are those associated with the use of either component alone. Most frequently reported were drowsiness, dry mouth, constipation, blurred vision, dizziness and bloating. Other side effects occurring less commonly included vivid dreams, impotence, tremor, confusion and nasal congestion. Many symptoms common to the depressive state, such as anorexia, fatigue, weakness, restlessness and lethargy, have been reported as side effects of treatment with both chlordiazepoxide and amitriptyline hydrochloride tablets and amitriptyline.
Granulocytopenia, jaundice and hepatic dysfunction of uncertain etiology have also been observed rarely with chlordiazepoxide and amitriptyline hydrochloride tablets. When treatment with chlordiazepoxide and amitriptyline hydrochloride tablets is prolonged, periodic blood counts and liver function tests are advisable.
Note
Included in the listing which follows are adverse reactions which have not been reported with chlordiazepoxide and amitriptyline hydrochloride. However, they are included because they have been reported during therapy with one or both of the components or closely related drugs.
Cardiovascular: Hypotension, hypertension, tachycardia, palpitations, myocardial infarction, arrhythmias, heart block, stroke.
Psychiatric: Euphoria, apprehension, poor concentration, delusions, hallucinations, hypomania and increased or decreased libido.
Neurologic: Incoordination, ataxia, numbness, tingling and paresthesias of the extremities, extrapyramidal symptoms, syncope, changes in EEG patterns.
Anticholinergic: Disturbance of accommodation, paralytic ileus, urinary retention, dilatation of urinary tract.
Allergic: Skin rash, urticaria, photosensitization, edema of face and tongue, pruritus.
Hematologic: Bone marrow depression including agranulocytosis, eosinophilia, purpura, thrombocytopenia.
Gastrointestinal: Nausea, epigastric distress, vomiting, anorexia, stomatitis, peculiar taste, diarrhea, black tongue.
Endocrine: Testicular swelling and gynecomastia in the male, breast enlargement, galactorrhea and minor menstrual irregularities in the female, elevation and lowering of blood sugar levels, and syndrome of inappropriate ADH (antidiuretic hormone) secretion.
Other: Headache, weight gain or loss, increased perspiration, urinary frequency, mydriasis, jaundice, alopecia, parotid swelling.
-
DRUG ABUSE AND DEPENDENCE
Controlled Substance
Chlordiazepoxide and amitriptyline hydrochloride tablets contain chlordiazepoxide, a Schedule IV controlled substance.
Abuse
Chlordiazepoxide and amitriptyline hydrochloride tablets are a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders (see WARNINGS: Abuse, Misuse, and Addiction).
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
Dependence
Physical Dependence
Chlordiazepoxide and amitriptyline hydrochloride tablets may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use (see WARNINGS: Dependence and Withdrawal Reactions).
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide and amitriptyline hydrochloride tablets or reduce the dosage (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Chlordiazepoxide and Amitriptyline Hydrochloride Tablets and WARNINGS: Dependence and Withdrawal Reactions).
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to chlordiazepoxide and amitriptyline hydrochloride tablets may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of chlordiazepoxide and amitriptyline hydrochloride tablets may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
-
OVERDOSAGE
Overdosage of chlordiazepoxide and amitriptyline hydrochloride tablets, which contain a benzodiazepine (chlordiazepoxide) and a tricyclic antidepressant (amitriptyline hydrochloride), may manifest signs and symptoms related to either of its components. Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose.
Critical manifestations of tricyclic antidepressant overdosage include cardiac dysrhythmias, severe hypotension, convulsions and CNS depression, including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity. Obtain an ECG and immediately initiate cardiac monitoring; hospital monitoring is required as soon as possible.
Other signs of overdosage may include confusion, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia or any of the symptoms listed under ADVERSE REACTIONS.
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal (see WARNINGS: Abuse, Misuse, and Addiction). Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures with chlordiazepoxide and amitriptyline hydrochloride tablets because overdosage with amitriptyline and other tricyclic and tetracyclic antidepressants increases the risk of seizures. Seizure risk is also increased in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use also may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus).
Contact the Poison Help line (1-800-222-1222) or medical toxicologist for additional overdosage management recommendations.
-
DOSAGE AND ADMINISTRATION
Optimum dosage varies with the severity of the symptoms and the response of the individual patient. When a satisfactory response is obtained, dosage should be reduced to the smallest amount needed to maintain the remission. The larger portion of the total daily dose may be taken at bedtime. In some patients, a single dose at bedtime may be sufficient. In general, lower dosages are recommended for elderly patients.
Chlordiazepoxide and amitriptyline hydrochloride tablets 10 mg/25 mg are recommended in an initial dosage of 3 or 4 tablets daily in divided doses; this may be increased to 6 tablets daily as required. Some patients respond to smaller doses and can be maintained on 2 tablets daily.
Chlordiazepoxide and amitriptyline hydrochloride tablets 5 mg/12.5 mg in an initial dosage of 3 or 4 tablets daily in divided doses may be satisfactory in patients who do not tolerate higher doses.
Screen for Bipolar Disorder Prior to Starting Chlordiazepoxide and Amitriptyline Hydrochloride Tablets
Prior to initiating treatment with chlordiazepoxide and amitriptyline hydrochloride tablets or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania (see WARNINGS: Activation of Mania or Hypomania).
Discontinuation or Dosage Reduction of Chlordiazepoxide and Amitriptyline Hydrochloride Tablets
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide and amitriptyline hydrochloride tablets or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see WARNINGS: Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE: Dependence).
-
HOW SUPPLIED
Chlordiazepoxide and Amitriptyline Hydrochloride Tablets, USP are available containing 5 mg of chlordiazepoxide, USP and 14 mg of amitriptyline hydrochloride, USP equivalent to 12.5 mg of amitriptyline or 10 mg of chlordiazepoxide, USP and 27.98 mg of amitriptyline hydrochloride, USP equivalent to 25 mg of amitriptyline.
The 5 mg/12.5 mg tablets are green, film-coated, round, unscored tablets debossed with MYLAN on one side of the tablet and 211 on the other side. They are available as follows:
NDC 0378-0211-01
bottles of 100 tabletsNDC 0378-0211-05
bottles of 500 tabletsThe 10 mg/25 mg tablets are white, film-coated, round, unscored tablets debossed with MYLAN on one side of the tablet and 277 on the other side. They are available as follows:
NDC 0378-0277-01
bottles of 100 tabletsNDC 0378-0277-05
bottles of 500 tabletsStore at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Store in a dry place.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
PHARMACIST: Dispense a Medication Guide with each prescription.
-
Medication Guide
Chlordiazepoxide and Amitriptyline Hydrochloride Tablets, USP CIV
(klorʺ dye azʺ e poxʹ ide amʺ i tripʹ ti leen hyeʺ droe klorʹ ide)What is the most important information I should know about chlordiazepoxide and amitriptyline hydrochloride tablets?
- •
-
Chlordiazepoxide is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:
- o
- shallow or slowed breathing
- o
- breathing stops (which may lead to the heart stopping)
- o
- excessive sleepiness (sedation)
- Do not drive or operate heavy machinery until you know how taking chlordiazepoxide and amitriptyline hydrochloride tablets with opioids affect you.
- •
-
Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines, including chlordiazepoxide and amitriptyline hydrochloride tablets, which can lead to overdose and serious side effects including coma and death.
- o
- Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including chlordiazepoxide and amitriptyline hydrochloride tablets. These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
- o
- You can develop an addiction even if you take chlordiazepoxide and amitriptyline hydrochloride tablets exactly as prescribed by your healthcare provider.
- o
- Take chlordiazepoxide and amitriptyline hydrochloride tablets exactly as your healthcare provider prescribed.
- o
- Do not share your chlordiazepoxide and amitriptyline hydrochloride tablets with other people.
- o
- Keep chlordiazepoxide and amitriptyline hydrochloride tablets in a safe place and away from children.
- •
-
Physical dependence and withdrawal reactions. Chlordiazepoxide and amitriptyline hydrochloride tablets can cause physical dependence and withdrawal reactions.
- o
- Do not suddenly stop taking chlordiazepoxide and amitriptyline hydrochloride tablets. Stopping chlordiazepoxide and amitriptyline hydrochloride tablets suddenly can cause serious and life-threatening side effects, including, unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms.
- o
- Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
- o
- Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- o
- Do not take more chlordiazepoxide and amitriptyline hydrochloride tablets than prescribed or take chlordiazepoxide and amitriptyline hydrochloride tablets for longer than prescribed.
- •
-
Increased risk of suicidal thoughts or actions in children, adolescents and young adults. Chlordiazepoxide and amitriptyline hydrochloride tablets and other antidepressant medicines may increase suicidal thoughts or actions in some people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed.
- o
- Depression or other serious mental illnesses are the most important cause of suicidal thoughts or actions. Some people may have a higher risk of having suicidal thoughts or actions. These include people who have (or have a family history of) depression, bipolar illness (also called manic-depressive illness), or a history of suicidal thoughts or actions.
-
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- o
- Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings or if you or your family member develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed.
- o
- Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings or if you or your family member develop suicidal thoughts or actions.
- o
- Keep all follow-up visits with your healthcare provider and call between visits if you are worried about symptoms.
- Call your healthcare provider right away if you or your family member have any of the following symptoms, especially if they are new, worse, or worry you:
- •
- thoughts about suicide or dying
- •
- new or worse depression
- •
- feeling agitated or restless
- •
- trouble sleeping
- •
- acting aggressive, being angry or violent
- •
- other unusual changes in behavior or mood
- •
- attempts to commit suicide
- •
- new or worse anxiety or irritability
- •
- an extreme increase in activity and talking
- •
- new or worse panic attacks
- •
- acting on dangerous impulses
What are chlordiazepoxide and amitriptyline hydrochloride tablets?
- •
- Chlordiazepoxide and amitriptyline hydrochloride tablets are a prescription medicine used to treat moderate to severe depression that can happen with moderate to severe anxiety.
- •
- Chlordiazepoxide and amitriptyline hydrochloride tablets are a federal controlled substance (C-IV) because they contain chlordiazepoxide that can be abused or lead to dependence. Keep chlordiazepoxide and amitriptyline hydrochloride tablets in a safe place to prevent misuse and abuse. Selling or giving away chlordiazepoxide and amitriptyline hydrochloride tablets may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
- •
- It is not known if chlordiazepoxide and amitriptyline hydrochloride tablets are safe and effective in children.
Do not take chlordiazepoxide and amitriptyline hydrochloride tablets if you:
- •
- are allergic to chlordiazepoxide and amitriptyline hydrochloride or any of the ingredients in chlordiazepoxide and amitriptyline hydrochloride tablets. See the end of this Medication Guide for a complete list of ingredients in chlordiazepoxide and amitriptyline hydrochloride tablets.
- •
- have had an allergic reaction to other benzodiazepines or tricyclic antidepressant medicines
- •
- take a medicine called a Monoamine Oxidase Inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI.
- •
- are recovering from a heart attack
Before you take chlordiazepoxide and amitriptyline hydrochloride tablets, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have or have a family history of suicide, bipolar disorder, or depression
- •
- have a history of drug or alcohol abuse or addiction
- •
- have eye problems
- •
- have problems urinating or emptying your bladder
- •
- have kidney or liver problems
- •
- have or had heart problems, including heart attack or a stroke
- •
- have or have had seizures
- •
- have a thyroid problem
- •
- plan to have surgery
- •
- receive electroconvulsive therapy (ECT)
- •
- are pregnant or plan to become pregnant.
- •
- Taking chlordiazepoxide and amitriptyline hydrochloride tablets late in pregnancy may cause your baby to have symptoms of sedation (breathing problems, sluggishness, low muscle tone), and/or withdrawal symptoms (jitteriness, irritability, restlessness, shaking, excessive crying, feeding problems).
- •
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with chlordiazepoxide and amitriptyline hydrochloride tablets.
- •
- There is a pregnancy registry for women who take chlordiazepoxide and amitriptyline hydrochloride tablets during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. If you become pregnant during treatment with chlordiazepoxide and amitriptyline hydrochloride tablets, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychiatric Medications. You can register by calling 1-866-961-2388 or visiting https://womansmentalhealth.org/pregnancyregistry/.
- •
- are breastfeeding or plan to breastfeed. It is not known if chlordiazepoxide and amitriptyline hydrochloride pass into your breastmilk.
- •
- Talk to your healthcare provider about the best way to feed your baby if you take chlordiazepoxide and amitriptyline hydrochloride tablets.
- •
- Breastfeeding is not recommended during treatment with chlordiazepoxide and amitriptyline hydrochloride tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking chlordiazepoxide and amitriptyline hydrochloride tablets with certain other medicines can cause side effects or affect how well chlordiazepoxide and amitriptyline hydrochloride tablets or the other medicines work.
Ask your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take chlordiazepoxide and amitriptyline hydrochloride tablets with your other medicines.
Do not start or stop any other medicines during treatment with chlordiazepoxide and amitriptyline hydrochloride tablets without talking to your healthcare provider first. Stopping chlordiazepoxide and amitriptyline hydrochloride tablets suddenly may cause you to have serious side effects. See, “What are the possible side effects of chlordiazepoxide and amitriptyline hydrochloride tablets?”
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take chlordiazepoxide and amitriptyline hydrochloride tablets?
- •
- Take chlordiazepoxide and amitriptyline hydrochloride tablets exactly as your healthcare provider tells you to take them.
- •
- If you take too many chlordiazepoxide and amitriptyline hydrochloride tablets, call your healthcare provider or go to the nearest emergency room right away.
What are the possible side effects of chlordiazepoxide and amitriptyline hydrochloride tablets?
Chlordiazepoxide and amitriptyline hydrochloride tablets may cause serious side effects, including:
- •
- See “What is the most important information I should know about chlordiazepoxide and amitriptyline hydrochloride tablets?”
- •
-
Chlordiazepoxide and amitriptyline hydrochloride tablets can make you sleepy or dizzy, and can slow your thinking and motor skills.
- o
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how chlordiazepoxide and amitriptyline hydrochloride tablets affect you.
- o
- Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking chlordiazepoxide and amitriptyline hydrochloride tablets without first talking to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, chlordiazepoxide and amitriptyline hydrochloride tablets may make your sleepiness or dizziness much worse.
The most common side effects of chlordiazepoxide and amitriptyline hydrochloride tablets include:
- •
- drowsiness
- •
- dizziness
- •
- blurred vision
- •
- constipation
- •
- dry mouth
- •
- bloating
These are not all the possible side effects of chlordiazepoxide and amitriptyline hydrochloride tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store chlordiazepoxide and amitriptyline hydrochloride tablets?
- •
- Store chlordiazepoxide and amitriptyline hydrochloride tablets at room temperature between 20° to 25°C (68° to 77°F).
- •
- Store in a dry place.
- •
- Keep chlordiazepoxide and amitriptyline hydrochloride tablets in a tightly closed, child-resistant container and out of the light.
Keep chlordiazepoxide and amitriptyline hydrochloride tablets and all medicines out of the reach of children.
General information about the safe and effective use of chlordiazepoxide and amitriptyline hydrochloride tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use chlordiazepoxide and amitriptyline hydrochloride tablets for a condition for which they were not prescribed. Do not give chlordiazepoxide and amitriptyline hydrochloride tablets to other people, even if they have the same symptoms that you have. They may harm them. You can ask your pharmacist or healthcare provider for information about chlordiazepoxide and amitriptyline hydrochloride tablets that is written for health professionals.
What are the ingredients in chlordiazepoxide and amitriptyline hydrochloride tablets?
Active ingredients: chlordiazepoxide, amitriptyline hydrochloride
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch (corn), sodium lauryl sulfate and titanium dioxide. In addition, the
5 mg/12.5 mg tablets also contain D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake and FD&C Yellow No. 6 Aluminum Lake and the 10 mg/25 mg tablets also contain polydextrose and triacetin.Manufactured for: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 U.S.A.
For more information, call Mylan at 1-877-446-3679 (1-877-4-INFO-RX).
The brands listed are trademarks of their respective owners.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Manufactured by:
ALPHAPHARM PTY LTD
15 Garnet Street
Carole Park QLD 4300
AustraliaRevised: 2/2024
ALP:CDAM:R6mmh/ALP:MG:CDAM:R5m/ALP:MG:CDAM:R5mh
(3452/5)
-
PRINCIPAL DISPLAY PANEL – 5 mg/12.5 mg
NDC 0378-0211-01
Chlordiazepoxide
and
Amitriptyline HCl
Tablets, USP
CIV
5 mg/
12.5 mg*Rx only 100 Tablets
*Each film-coated tablet contains
5 mg of chlordiazepoxide, USP and
14 mg of amitriptyline hydrochloride,
USP equivalent to 12.5 mg of
amitriptyline.Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.Keep container tightly closed.
Keep this and all medication
out of the reach of children.Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]Store in a dry place.
Usual Dosage: See
accompanying prescribing
information.PHARMACIST: Dispense the
accompanying Medication
Guide to each patient.Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Made in Australia
3448/0
RALP0211A
-
PRINCIPAL DISPLAY PANEL – 10 mg/25 mg
NDC 0378-0277-01
Chlordiazepoxide
and
Amitriptyline HCl
Tablets, USP
CIV
10 mg/
25 mg*Rx only 100 Tablets
*Each film-coated tablet contains
10 mg of chlordiazepoxide, USP and
27.98 mg of amitriptyline hydrochloride,
USP equivalent to 25 mg of
amitriptyline.Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.Keep container tightly closed.
Keep this and all medication
out of the reach of children.Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]Store in a dry place.
Usual Dosage: See
accompanying prescribing
information.PHARMACIST: Dispense the
accompanying Medication
Guide to each patient.Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Made in Australia
3450/0
RALP0277A
-
INGREDIENTS AND APPEARANCE
CHLORDIAZEPOXIDE AND AMITRIPTYLINE HYDROCHLORIDE
chlordiazepoxide and amitriptyline hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0378-0211 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORDIAZEPOXIDE (UNII: 6RZ6XEZ3CR) (CHLORDIAZEPOXIDE - UNII:6RZ6XEZ3CR) CHLORDIAZEPOXIDE 5 mg AMITRIPTYLINE HYDROCHLORIDE (UNII: 26LUD4JO9K) (AMITRIPTYLINE - UNII:1806D8D52K) AMITRIPTYLINE HYDROCHLORIDE 14 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) FD&C BLUE NO. 1--ALUMINUM LAKE (UNII: J9EQA3S2JM) Product Characteristics Color GREEN Score no score Shape ROUND Size 10mm Flavor Imprint Code MYLAN;211 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0378-0211-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/10/1986 2 NDC:0378-0211-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/10/1986 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA071297 12/10/1986 CHLORDIAZEPOXIDE AND AMITRIPTYLINE HYDROCHLORIDE
chlordiazepoxide and amitriptyline hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0378-0277 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORDIAZEPOXIDE (UNII: 6RZ6XEZ3CR) (CHLORDIAZEPOXIDE - UNII:6RZ6XEZ3CR) CHLORDIAZEPOXIDE 10 mg AMITRIPTYLINE HYDROCHLORIDE (UNII: 26LUD4JO9K) (AMITRIPTYLINE - UNII:1806D8D52K) AMITRIPTYLINE HYDROCHLORIDE 27.98 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYDEXTROSE (UNII: VH2XOU12IE) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color WHITE Score no score Shape ROUND Size 10mm Flavor Imprint Code MYLAN;277 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0378-0277-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/10/1986 2 NDC:0378-0277-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/10/1986 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA071297 12/10/1986 Labeler - Mylan Pharmaceuticals Inc. (059295980)