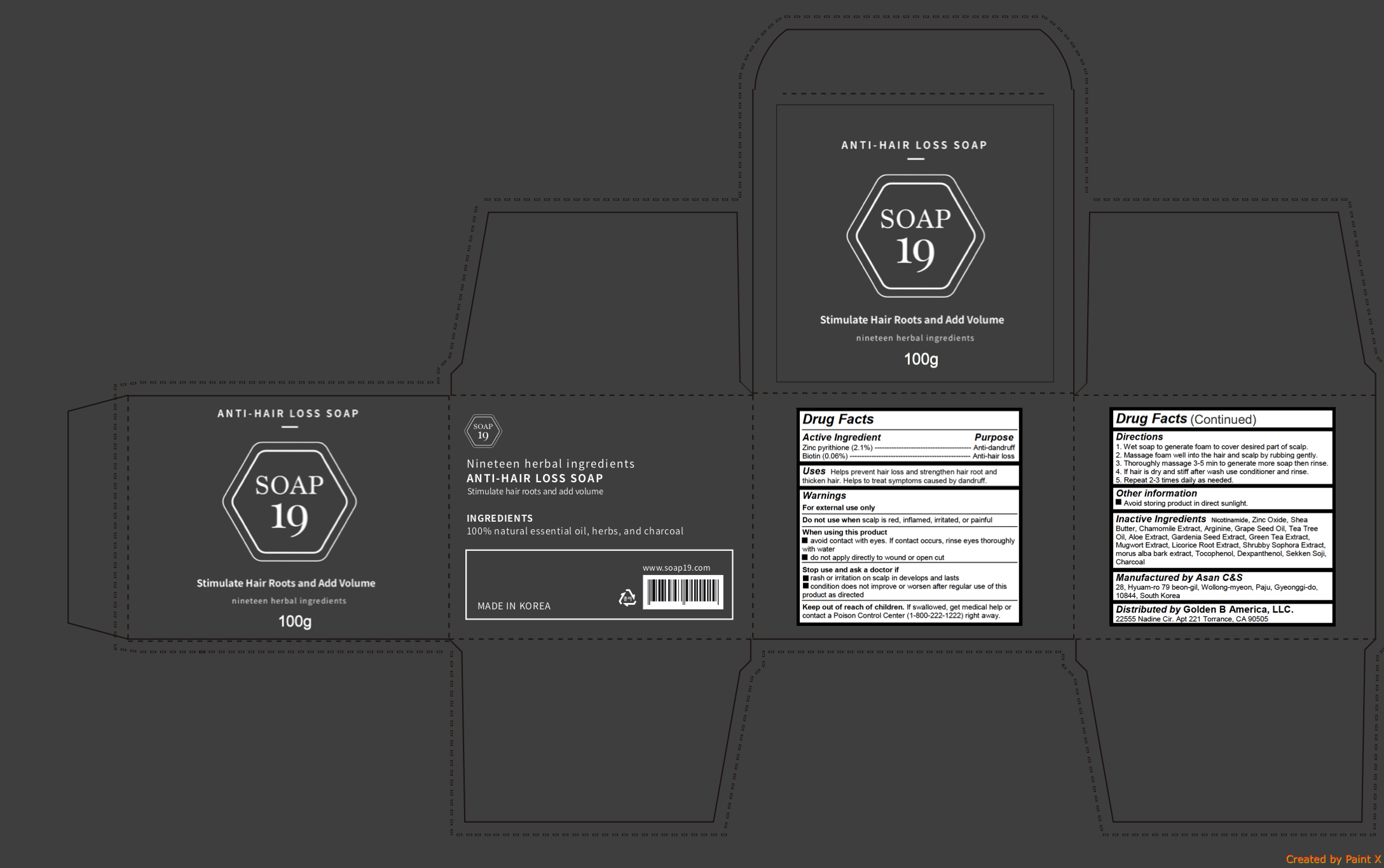
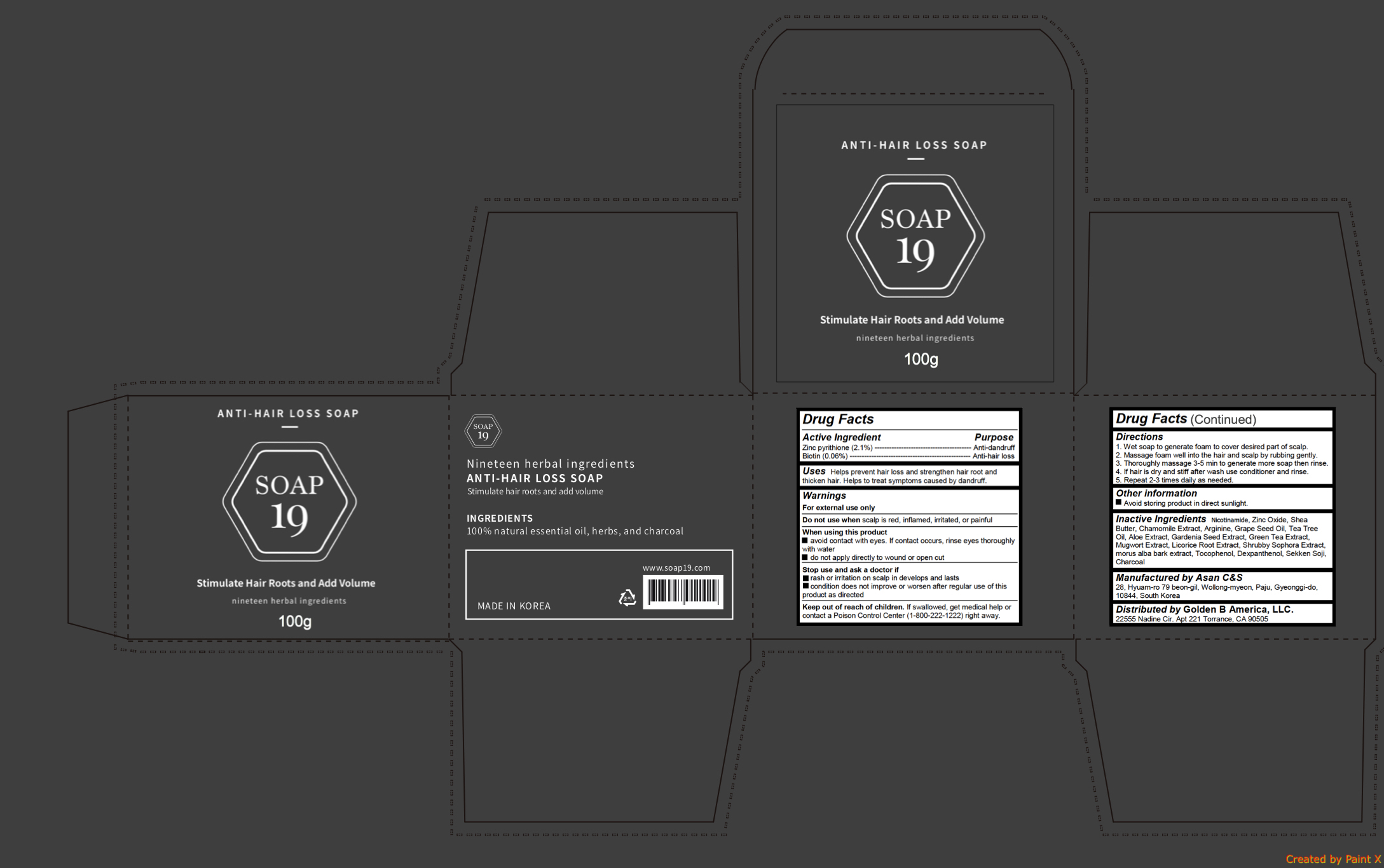
Label: ANTI HAIR LOSS- pyrithione zinc, biotin soap
-
Contains inactivated NDC Code(s)
NDC Code(s): 72100-100-01, 72100-100-02 - Packager: Golden B America, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 1, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use when scalp is red, inflamed, irritated, or painful
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
- do not apply directly to wound or open cut
Stop use and ask a doctor if
- rash or irritation on scalp in develops and lasts
- condition does not improve or worsen after regular use of this product as directed
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- Directions
- Keep out of reach of children
- Inactive ingredients
- Anti Hair Loss Soap
-
INGREDIENTS AND APPEARANCE
ANTI HAIR LOSS
pyrithione zinc, biotin soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72100-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 2.1 g in 100 g BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 0.06 g in 100 g Inactive Ingredients Ingredient Name Strength SHEA BUTTER (UNII: K49155WL9Y) CHAMOMILE (UNII: FGL3685T2X) ARGININE (UNII: 94ZLA3W45F) NIACINAMIDE (UNII: 25X51I8RD4) ZINC OXIDE (UNII: SOI2LOH54Z) GRAPE SEED OIL (UNII: 930MLC8XGG) TEA TREE OIL (UNII: VIF565UC2G) ALOE (UNII: V5VD430YW9) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ARTEMISIA PRINCEPS LEAF (UNII: SY077EW02G) LICORICE (UNII: 61ZBX54883) MORUS ALBA BARK (UNII: 7O71A48NDP) TOCOPHEROL (UNII: R0ZB2556P8) DEXPANTHENOL (UNII: 1O6C93RI7Z) ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72100-100-02 1 in 1 PACKAGE 03/13/2018 1 NDC:72100-100-01 100 g in 1 CELLO PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/13/2018 Labeler - Golden B America, LLC. (081015296) Registrant - Golden B America, LLC. (081015296) Establishment Name Address ID/FEI Business Operations Golden B America, LLC. 081015296 relabel(72100-100) Establishment Name Address ID/FEI Business Operations Asan C&s 631139649 manufacture(72100-100)