Label: CLINISOL- lysine, leucine, phenylalanine, valine, histidine, isoleucine, methionine, threonine, tryptophan, alanine, arginine, glycine, proline, glutamic acid, serine, aspartic acid, tyrosine injection, solution
- NDC Code(s): 0338-0502-03, 0338-0502-06
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated June 1, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package is a sterile, clear, nonpyrogenic, hypertonic solution of essential and nonessential amino acids. A Pharmacy Bulk Package is a container of a sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous infusion.
The VIAFLEX plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). Exposure to temperatures above 25ºC/77ºF during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million; however, the safety of the plastic has been confirmed in tests in animals according to USP biological test for plastic containers as well as by tissue culture toxicity studies.
Each 100 mL of 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package contains:
Amino Acids
Total Nitrogen
pH15.0 g
2.37 g
6.0 (5.0 to 7.0)
(pH adjusted with glacial acetic acid)
Essential Amino Acids
Lysine - (from Lysine Acetate) C6H14N2O2
Leucine - C6H13NO2
Phenylalanine – C9H11NO2
Valine - C5H11NO2
Histidine - C6H9N3O2
Isoleucine - C6H13NO2
Methionine - C5H11NO2S
Threonine - C4H9NO3
Tryptophan - C11H12N2O2
Nonessential Amino Acids
Alanine - C3H7NO2
Arginine - C6H14N4O2
Glycine - C2H5NO2
Proline - C5H9NO2
Glutamic Acid - C5H9NO4
Serine – C3H7NO3
Aspartic Acid – C4H7NO4
Tyrosine – C9H11NO3
Anion profiles per liter*
Acetate from Lysine Acetate and glacial acetic acic
*Balanced by ions from amino acids
Osmolarity (Calc.)
1.18 g
1.04 g
1.04 g
960 mg
894 mg
749 mg
749 mg
749 mg
250 mg
2.17 g
1.47 g
1.04 g
894 mg
749 mg
592 mg
434 mg
39 mg
127 mEq
1357 mOsmol/L
-
CLINICAL PHARMACOLOGY
15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package administered parenterally will provide biologically utilizable source material for protein synthesis when used with concentrated calorie sources, electrolytes, vitamins and minerals.
Central Infusion
15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package is intended for use in a pharmacy admixture program and as such is restricted to the preparation of admixtures for intravenous use. 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package given by central venous infusion in combination with energy sources, vitamins, trace elements and electrolytes, will meet the requirements for weight maintenance or weight gain. The energy component may be derived solely from dextrose or may be provided by a combination of dextrose and intravenous fat emulsion.
-
INDICATIONS AND USAGE
15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package is indicated as an adjunct in the offsetting of nitrogen loss or in the treatment of negative nitrogen balance in patients where: (1) the alimentary tract cannot or should not be used, (2) gastrointestinal absorption of protein is impaired, or (3) metabolic requirements for protein are substantially increased, as with extensive burns.
- CONTRAINDICATIONS
-
WARNINGS
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Because of the potential for life-threatening events, caution should be taken to ensure that precipitates have not formed in any parenteral nutrient admixture.
This injection is for compounding only, not for direct infusion.
Once container closure has been penetrated, withdrawal of contents should be completed within 4 hours. After initial entry, maintain contents at room temperature (25ºC/77ºF).
Any admixture storage should be under refrigeration and limited to a brief period of time, preferably less than 24 hours.
Administration of amino acid solutions at excessive rates or to patients with hepatic insufficiency may result in plasma amino acid imbalances, hyperammonemia, prerenal azotemia, stupor and coma. Conservative doses of amino acids should be given to these patients, dictated by the nutritional status of the patient. Should symptoms of hyperammonemia develop, amino acid administration should be discontinued and the patient's clinical status reevaluated.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 μg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
General
In order for parenterally administered amino acids to be retained by the body and utilized for protein synthesis adequate calories must be administered concurrently.
The administration of 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package as part of total parenteral nutrition (TPN) with large volumes of hyperosmotic fluids requires periodic monitoring of the patient for signs of hyperosmolarity, hyperglycemia, glycosuria and hypertriglyceridemia.
During parenteral nutrition with concentrated dextrose and amino acid solutions, essential fatty acid deficiency syndrome may develop but may not be clinically apparent. Early demonstration of this condition can only be accomplished by analysis of plasma lipids. The syndrome may be prevented or corrected by appropriate treatment with intravenous fat emulsions.
For complete nutritional support, TPN regimens must also include multiple vitamins and trace elements. Potentially incompatible ions such as calcium and phosphate may be added to alternate infusate containers to avoid precipitation.
Initiation and termination of infusions of TPN fluids must be gradual to permit adjustment of endogenous insulin release.
Caution should be exercised against volume overload.
Do not administer unless solution is clear.
TPN delivered through a central or large peripheral vein is a special technique requiring a team effort by physician, nurse and pharmacist. The responsibility for administering this therapy should be confined to those trained in the procedures and alert to signs of complications. Complications known to occur from the placement of central venous catheters include sepsis and vein irritation due to hypertonicity of the infused solution. The risk of sepsis is present during intravenous therapy, especially when using central venous catheters for prolonged periods. It is imperative that the preparation of admixtures and the placement and care of the catheters be accomplished under controlled aseptic conditions. It is essential that a carefully prepared protocol, based on current medical practices be followed.
Drug product contains no more than 25 μg/L of aluminum.
Laboratory Tests
Frequent clinical evaluations and laboratory determinations are necessary for proper monitoring during administration.
Laboratory tests should include blood glucose, serum electrolytes, liver and renal function, serum osmolarity, blood ammonia, serum protein, pH, hematocrit, and WBC. When 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package is combined with electrolytes, care should be used in administering this solution to patients with congestive heart failure, renal failure, edema, adrenal hyperactivity, acid base imbalance and those receiving diuretics or antihypertensive therapy. Serum electrolytes should be monitored daily.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Long term animal studies with 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package have not been performed to evaluate the carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy:
Teratogenic Effects
Animal reproduction studies have not been conducted with 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package. It is also not known whether 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
Caution should be exercised when 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package is administered to a nursing woman.
Pediatric Use:
Safety and effectiveness of 15% CLINISOL - sulfite-free (Amino Acid) Injection in pediatric patients have not been established by adequate and well-controlled studies. However, the use of amino acid injections in pediatric patients as an adjunct in the offsetting of nitrogen loss or in the treatment of negative nitrogen balance is referenced in the medical literature. See Dosage and Administration.
-
ADVERSE REACTIONS
Local reactions consisting of a warm sensation, erythema, phlebitis and thrombosis at the infusion site have occurred with peripheral intravenous infusion of amino acids. In such cases the infusion site should be changed promptly to another vein. Generalized flushing, fever and nausea also have been reported during peripheral infusions of amino acid solutions.
The following metabolic complications have been reported with administration of TPN: metabolic acidosis and alkalosis, hypophosphatemia, hypocalcemia, osteoporosis, glycosuria, hyperglycemia, hyperosmolar nonketotic states and dehydration, rebound hypoglycemia, osmotic diuresis and dehydration, elevated liver enzymes, hypo- and hypervitaminosis, electrolyte imbalances, hyperammonemia, coma and death.
Sepsis has been reported following intravenous therapy, especially when using central venous catheters for prolonged periods.
Complications known to occur from the placement of central venous catheters are pneumothorax, hemothorax, hydrothorax, artery puncture and transection injury to the brachial plexus, malposition of the catheter, formation of arteriovenous fistula, phlebitis, thrombosis and air and catheter emboli.
-
DOSAGE AND ADMINISTRATION
Doses which achieve nitrogen equilibrium or positive balance are the most desirable. The dosage on the first day should be approximately half the anticipated optimal dosage and should be increased gradually to minimize glycosuria; similarly, withdrawal should be accomplished gradually to avoid rebound hypoglycemia.
Fat emulsion coadministration should be considered when prolonged (more than 5 days) parenteral nutrition is required in order to prevent essential fatty acid deficiency (EFAD).
Pediatric Use:
Use of 15% CLINISOL - sulfite-free (Amino Acid) Injection in pediatric patients is governed by the same considerations that affect the use of any amino acid solution in pediatrics. The amount administered is dosed on the basis of grams of amino acids/kg of body weight/day. Two to three g/kg of body weight for infants with adequate calories are generally sufficient to satisfy protein needs and promote positive nitrogen balance. Solutions administered by peripheral vein should not exceed twice normal serum osmolarity (718 mOsmol/L).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
A slight yellow color does not alter the quality and efficacy of this product.
Additives may be incompatible. Complete information is not available. Those additives known to be incompatible should not be used. Consult with pharmacist, if available. When compounding admixtures, use aseptic technique. Mix thoroughly. Do not store any unused portion of 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package.
Central Vein Infusion
In unstressed adult patients with no unusual nitrogen losses, a minimum dosage of 0.1 gram nitrogen (4.2 mL of 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package) plus 4.4 grams (15 calories) of dextrose/fat emulsion per kilogram of body weight per day is required to achieve nitrogen balance and weight stability. For patients stressed by surgery, trauma or sepsis, and those with unusual nitrogen losses, the dosage required for maintenance may be as high as 0.3 to 0.4 grams of nitrogen (13 to 17 mL 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package) per kilogram of body weight per day, with proportionate increases in non-protein calories. Periodic assessment of nitrogen balance of the individual patient is the best indicator of proper dosage. Use of an infusion pump is advisable to maintain a steady infusion rate during central venous infusion.
Peripheral Infusion
In patients for whom central vein catheterization is not advisable, admixtures with 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package can be administered by peripheral vein. Dilution of 250 mL 15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package in 750 mL of 10% dextrose will reduce the osmolarity to a level (718 mOsmol/L) which is more favorable to the maintenance of the integrity of the walls of the veins. If infused simultaneously, fat emulsion will provide a dilution effect upon the osmolarity, as well. In pediatric patients, the final solution should not exceed twice normal serum osmolarity (718 mOsmol/L).
-
DIRECTIONS FOR USE OF VIAFLEX PLASTIC PHARMACY BULK PACKAGE CONTAINER
Do not use if overpouch has been previously opened or damaged.
To Open
Tear overpouch at notch and remove solution container. Visually inspect the container. If the Admin port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Some opacity of the plastic may be observed and will diminish gradually. Check for minute leaks by squeezing inner bag firmly. If leaks are found, discard.
Preparation for Admixing
- 1.
- The Pharmacy Bulk Package is to be used only in a suitable aseptic work area.
- 2.
- Suspend container.
- 3.
- Remove plastic protector from port.
- 4.
- Attach a suitable sterile transfer device or dispensing set which allows measured dispensing of the contents. Refer to complete directions accompanying device.
VIAFLEX containers should not be written on directly since ink migration has not been investigated. Affix accompanying label for date and time of entry.
-
HOW SUPPLIED
15% CLINISOL - sulfite-free (Amino Acid) Injection Pharmacy Bulk Package is available in VIAFLEX plastic containers as follows.
2B6187
500 mL
NDC 0338-0502-03
2B6189
2000 mL
NDC 0338-0502-06
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended the product be stored at room temperature (25ºC/77ºF).
- SPL UNCLASSIFIED SECTION
-
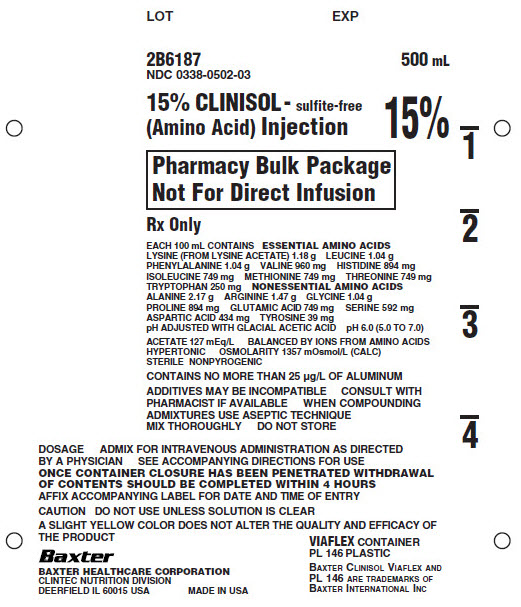
PRINCIPAL DISPLAY PANEL
LOT
EXP
2B6187
NDC 0338-0502-03500mL
15% CLINISOL - sulfite-free
(Amino Acid) Injection15%
Pharmacy Bulk Package
Not For Direct InfusionRx Only
EACH 100mL CONTAINS ESSENTIAL AMINO ACIDS
LYSINE (FROM LYSINE ACETATE) 1.18 g LEUCINE 1.04 g
PHENYLALANINE 1.04 g VALINE 960 mg HISTIDINE 894 mg
ISOLEUCINE 749 mg METHIONINE 749 mg THREONINE 749 mg
TRYPTOPHAN 250 mg NONESSENTIAL AMINO ACIDS
ALANINE 2.17 g ARGININE 1.47 g GLYCINE 1.04 g
PROLINE 894 mg GLUTAMIC ACID 749 mg SERINE 592 mg
ASPARTIC ACID 434 mg TYROSINE 39 mg
pH ADJUSTED WITH GLACIAL ACETIC ACID pH 6.0 (5.0 TO 7.0)ACETATE 127 mEq/L BALANCED BY IONS FROM AMINO ACIDS
HYPERTONIC OSMOLARITY 1357 mOsmol/L (CALC)
STERILE NONPYROGENICCONTAINS NO MORE THAN 25µg/L OF ALUMINUM
ADDITIVES MAY BE INCOMPATIBLE CONSULT WITH
PHARMACIST IF AVAILABLE WHEN COMPOUNDING
ADMIXTURES USE ASEPTIC TECHIQUE
MIX THOROUGHLY DO NOT STOREDOSAGE ADMIX FOR INTRAVEOUS ADMINISTRATION AS DIRECTED
BY A PHYSICIAN SEE ACCOMPANYING DIRECTIONS FOR USE
ONCE CONTAINER CLOSURE HAS BEEN PENETRATED WITHDRAWAL
OF CONTENTS SHOULD BE COMPLETED WITHIN 4 HOURS
AFFIX ACCOMPANYING LABEL FOR DATE AND TIME OF ENTRYCAUTION DO NOT USE UNLESS SOLUTION IS CLEAR
A SLIGHT YELLOW COLOR DOES NOT ALTER THE QUALITY AND EFFICACY OF
THE PRODUCTBaxter
BAXTER HEALTHCARE CORPORATION
CLINTEC NUTRITION DIVISION
DEERFIELD IL 60015 USA
MADE IN THE USAVIAFLEX CONTAINER
PL 146 PLASTICBAXTER CLINISOL VIAFLEX AND
PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INC1
2
3
4
-
INGREDIENTS AND APPEARANCE
CLINISOL
lysine, leucine, phenylalanine, valine, histidine, isoleucine, methionine, threonine, tryptophan, alanine, arginine, glycine, proline, glutamic acid, serine, aspartic acid, tyrosine injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0502 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LYSINE ACETATE (UNII: TTL6G7LIWZ) (LYSINE - UNII:K3Z4F929H6) LYSINE 1.18 g in 100 mL LEUCINE (UNII: GMW67QNF9C) (LEUCINE - UNII:GMW67QNF9C) LEUCINE 1.04 g in 100 mL PHENYLALANINE (UNII: 47E5O17Y3R) (PHENYLALANINE - UNII:47E5O17Y3R) PHENYLALANINE 1.04 g in 100 mL VALINE (UNII: HG18B9YRS7) (VALINE - UNII:HG18B9YRS7) VALINE 960 mg in 100 mL HISTIDINE (UNII: 4QD397987E) (HISTIDINE - UNII:4QD397987E) HISTIDINE 894 mg in 100 mL ISOLEUCINE (UNII: 04Y7590D77) (ISOLEUCINE - UNII:04Y7590D77) ISOLEUCINE 749 mg in 100 mL METHIONINE (UNII: AE28F7PNPL) (METHIONINE - UNII:AE28F7PNPL) METHIONINE 749 mg in 100 mL THREONINE (UNII: 2ZD004190S) (THREONINE - UNII:2ZD004190S) THREONINE 749 mg in 100 mL TRYPTOPHAN (UNII: 8DUH1N11BX) (TRYPTOPHAN - UNII:8DUH1N11BX) TRYPTOPHAN 250 mg in 100 mL ALANINE (UNII: OF5P57N2ZX) (ALANINE - UNII:OF5P57N2ZX) ALANINE 2.17 g in 100 mL ARGININE (UNII: 94ZLA3W45F) (ARGININE - UNII:94ZLA3W45F) ARGININE 1.47 g in 100 mL GLYCINE (UNII: TE7660XO1C) (GLYCINE - UNII:TE7660XO1C) GLYCINE 1.04 g in 100 mL PROLINE (UNII: 9DLQ4CIU6V) (PROLINE - UNII:9DLQ4CIU6V) PROLINE 894 mg in 100 mL GLUTAMIC ACID (UNII: 3KX376GY7L) (GLUTAMIC ACID - UNII:3KX376GY7L) GLUTAMIC ACID 749 mg in 100 mL SERINE (UNII: 452VLY9402) (SERINE - UNII:452VLY9402) SERINE 592 mg in 100 mL ASPARTIC ACID (UNII: 30KYC7MIAI) (ASPARTIC ACID - UNII:30KYC7MIAI) ASPARTIC ACID 434 mg in 100 mL TYROSINE (UNII: 42HK56048U) (TYROSINE - UNII:42HK56048U) TYROSINE 39 mg in 100 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0502-03 500 mL in 1 BAG; Type 0: Not a Combination Product 08/30/1996 2 NDC:0338-0502-06 2000 mL in 1 BAG; Type 0: Not a Combination Product 08/30/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA020512 08/30/1996 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 189326168 ANALYSIS(0338-0502) , LABEL(0338-0502) , MANUFACTURE(0338-0502) , PACK(0338-0502) , STERILIZE(0338-0502) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0502) Establishment Name Address ID/FEI Business Operations Baxter SA 370353835 ANALYSIS(0338-0502) , MANUFACTURE(0338-0502) , LABEL(0338-0502) , PACK(0338-0502) , STERILIZE(0338-0502)