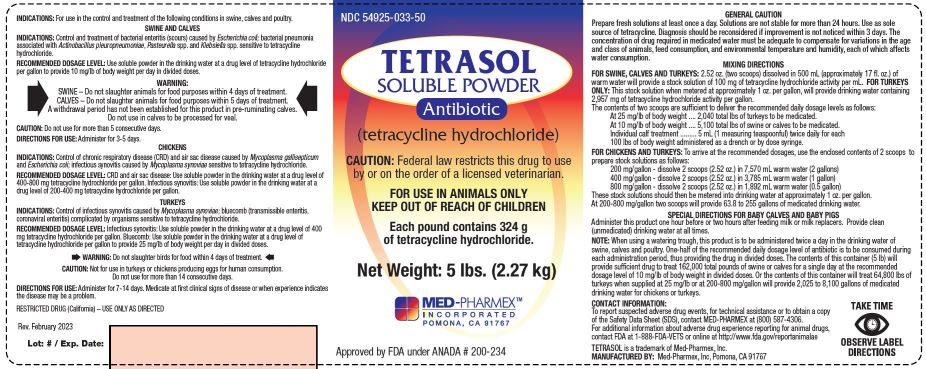
Label: TETRASOL- tetracycline hydrochloride powder, for solution
- NDC Code(s): 54925-033-50
- Packager: Med-Pharmex, Inc
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated February 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
(tetracycline hydrochloride)
Antibiotic
Approved by FDA under ANADA # 200-234
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
FOR USE IN ANIMALS ONLY - - KEEP OUT OF REACH OF CHILDREN
Each pound contains 324 g of tetracycline hydrochloride.
Net Wt.: 5 lbs. (2.27 kg) -
INDICATIONS FOR USE:
For use in the control and treatment of the following conditions in swine, calves and poultry.
SWINE AND CALVES
INDICATIONS: Control and treatment of bacterial enteritis (scours) caused by Escherichia coli; bacterial pneumonia associated with Actinobacillus pleuropneumoniae, Pasteurella spp. and Klebsiella spp. sensitive to tetracycline hydrochloride.
RECOMMENDED DOSAGE LEVEL: Use soluble powder in the drinking water at a drug level of tetracycline hydrochloride per gallon to provide 10 mg/lb of body weight per day in divided doses.
CAUTION: Do not use for more than 5 consecutive days.
DIRECTIONS FOR USE: Administer for 3-5 days._________________________
CHICKENS
INDICATIONS: Control of chronic respiratory disease (CRD) and air sac disease caused by Mycoplasma gallisepticum and Escherichia coli; infectious synovitis caused by Mycoplasma synoviae sensitive to tetracycline hydrochloride.
RECOMMENDED DOSAGE LEVEL: CRD and air sac disease: Use soluble powder in the drinking water at a drug level of 400-800 mg tetracycline hydrochloride per gallon. Infectious synovitis: Use soluble powder in the drinking water at a drug level of 200-400 mg tetracycline hydrochloride per gallon.
_________________________
TURKEYS
INDICATIONS: Control of infectious synovitis caused by Mycoplasma synoviae; bluecomb (transmissible enteritis, coronaviral enteritis) complicated by organisms sensitive to tetracycline hydrochloride.
RECOMMENDED DOSAGE LEVEL: Infectious synovitis: Use soluble powder in the drinking water at a drug level of 400 mg tetracycline hydrochloride per gallon. Bluecomb: Use soluble powder in the drinking water at a drug level of tetracycline hydrochloride per gallon to provide 25 mg/lb of body weight per day in divided doses.
CAUTION: Not for use in turkeys or chickens producing eggs for human consumption. Do not use for more than 14 consecutive days.
DIRECTIONS FOR USE: Administer for 7-14 days. Medicate at first clinical sign of disease or when experience indicates the disease may be a problem. -
WARNINGS:
SWINE – Do not slaughter animals for food purposes within 4 days of treatment.
____
CALVES – Do not slaughter animals for food purposes within 5 days of treatment. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal.
____
POULTRY - Do not slaughter birds for food within 4 days of treatment.
-
GENERAL CAUTION:
Prepare fresh solutions at least once a day. Solutions are not stable for more than 24 hours. Use as sole source of tetracycline. Diagnosis should be reconsidered if improvement is not noticed within 3 days. The concentration of drug required in medicated water must be adequate to compensate for variations in the age and class of animals, feed consumption, and environmental temperature and humidity, each of which affects water consumption.
-
DOSAGE AND ADMINISTRATION:
MIXING DIRECTIONS
FOR SWINE, CALVES AND TURKEYS: 2.52 oz. (two scoops) dissolved in 500 mL (approximately 17 fl. oz.) of warm water will provide a stock solution of 100 mg of tetracycline hydrochloride activity per mL.
FOR TURKEYS ONLY: This stock solution when metered at approximately 1 oz. per gallon, will provide drinking water containing 2,957 mg of tetracycline hydrochloride activity per gallon.
The contents of two scoops are sufficient to deliver the recommended daily dosage levels as follows:
At 25 mg/lb of body weight .... 2,040 total lbs of turkeys to be medicated.
At 10 mg/lb of body weight .... 5,100 total lbs of swine or calves to be medicated.
Individual calf treatment ......... 5 mL (1 measuring teaspoonful) twice daily for each 100 lbs of body weight administered as a drench or by dose syringe.FOR CHICKENS AND TURKEYS: To arrive at the recommended dosages, use the enclosed contents of 2 scoops to prepare stock solutions as follows:
200 mg/gallon - dissolve 2 scoops (2.52 oz.) in 7,570 mL warm water (2 gallons)
400 mg/gallon - dissolve 2 scoops (2.52 oz.) in 3,785 mL warm water (1 gallon)
800 mg/gallon - dissolve 2 scoops (2.52 oz.) in 1,892 mL warm water (0.5 gallon)
These stock solutions should then be metered into drinking water at approximately 1 oz. per gallon.
At 200-800 mg/gallon two scoops will provide 63.8 to 255 gallons of medicated drinking water.SPECIAL DIRECTIONS FOR BABY CALVES AND BABY PIGS - Net Weight: 5 lbs. (2.27 kg)
Administer this product one hour before or two hours after feeding milk or milk replacers. Provide clean (unmedicated) drinking water at all times.
NOTE: When using a watering trough, this product is to be administered twice a day in the drinking water of swine, calves and poultry. One-half of the recommended daily dosage level of antibiotic is to be consumed during each administration period, thus providing the drug in divided doses. The contents of this container (5 lbs) will provide sufficient drug to treat 162,000 total pounds of swine or calves for a single day at the recommended dosage level of 10 mg/lb of body weight in divided doses. Or the contents of this container will treat 64,800 lbs of turkeys when supplied at 25 mg/lb or at 200-800 mg/gallon will provide 2,025 to 8,100 gallons of medicated drinking water for chickens or turkeys.RESTRICTED DRUG (California) – USE ONLY AS DIRECTED
TAKE TIME OBSERVE LABEL DIRECTIONS
-
HOW SUPPLIED
CONTACT INFORMATION:
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Med-Pharmex at (800) 587-4306. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalaeManufactured by:
Med-Pharmex, Inc.
Pomona, CA 91767Rev. Feb. 2024
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TETRASOL
tetracycline hydrochloride powder, for solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54925-033 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACYCLINE HYDROCHLORIDE (UNII: P6R62377KV) (TETRACYCLINE - UNII:F8VB5M810T) TETRACYCLINE HYDROCHLORIDE 714 g in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54925-033-50 2.27 kg in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200234 07/22/1997 Labeler - Med-Pharmex, Inc (025353699)