Label: LOPERAMIDE HYDROCHLORIDE- loperamide hcl suspension
- NDC Code(s): 0363-0645-26, 0363-0645-34
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 19, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 7.5 mL)
- Purpose
- Use
-
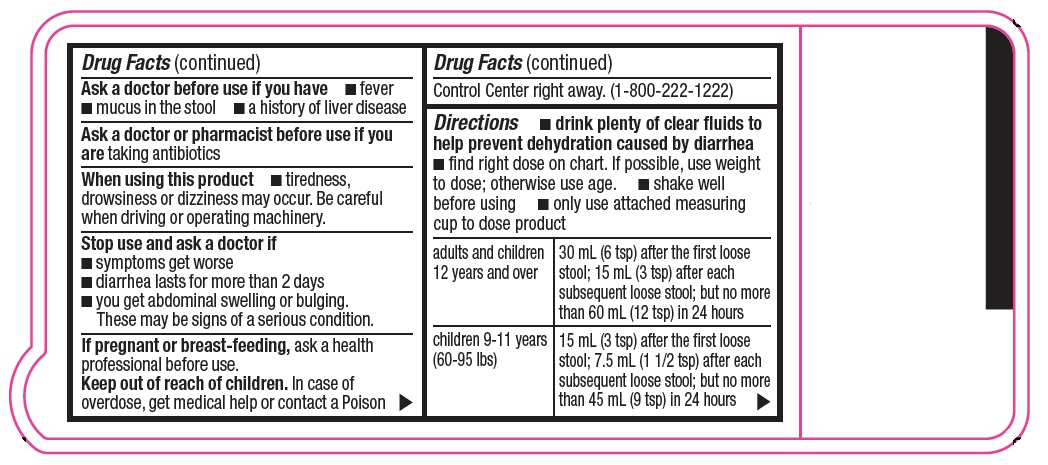
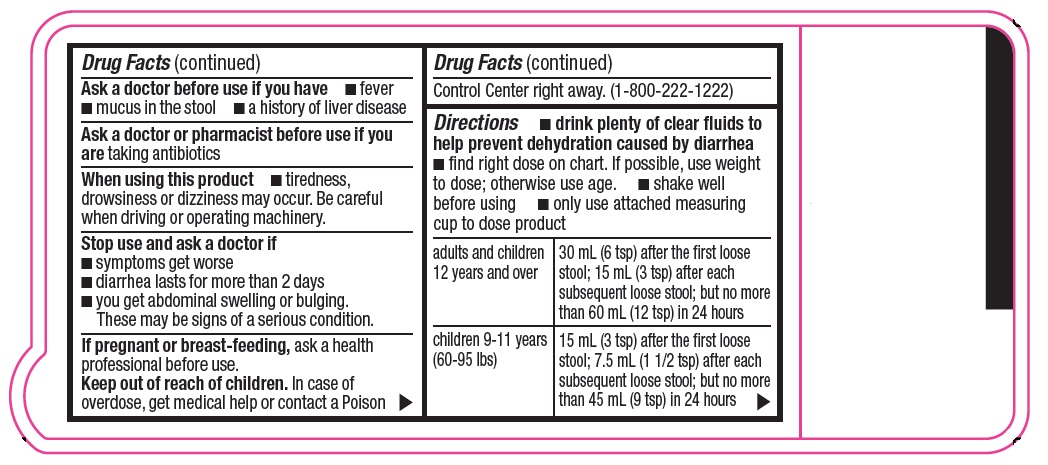
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl
When using this product
- •
- tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
-
Directions
- •
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- •
- find right dose on chart. If possible, use weight to dose; otherwise use age.
- •
- shake well before using
- •
- only use attached measuring cup to dose product
adults and children
12 years and over
30 mL (6 tsp) after the first loose stool; 15 mL (3 tsp) after each subsequent loose stool; but no more than 60 mL (12 tsp) in 24 hours
children 9-11 years
(60-95 lbs)
15 mL (3 tsp) after the first loose stool; 7.5 mL (1 1/2 tsp) after each subsequent loose stool; but no more than 45 mL (9 tsp) in 24 hours
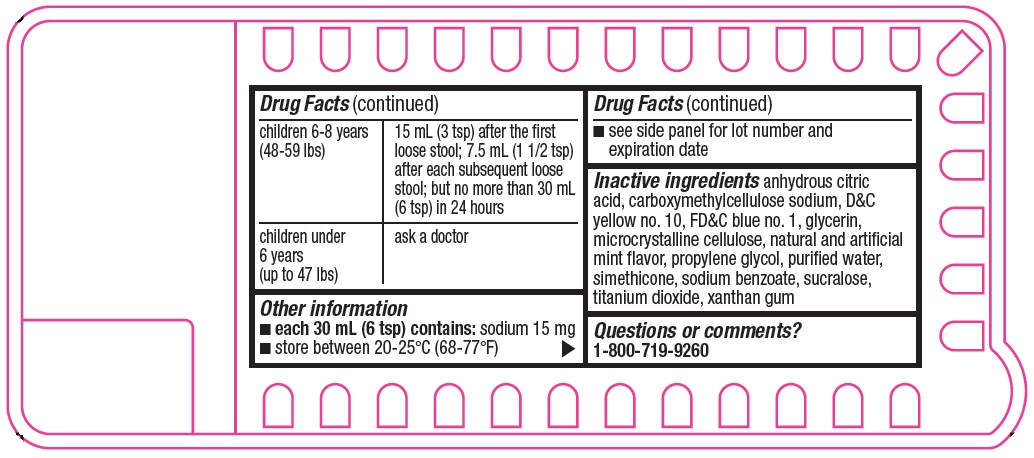
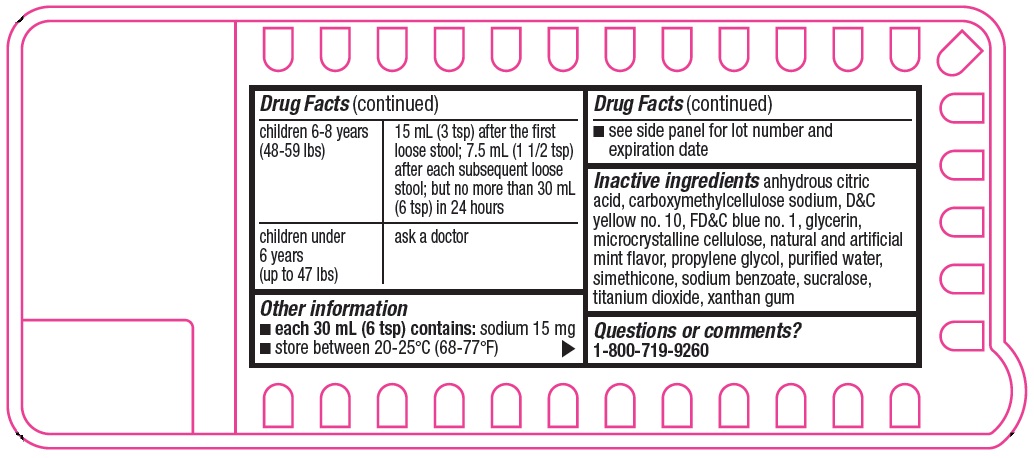
children 6-8 years
(48-59 lbs)
15 mL (3 tsp) after the first loose stool; 7.5 mL (1 1/2 tsp) after each subsequent loose stool; but no more than 30 mL (6 tsp) in 24 hours
children under
6 years
(up to 47 lbs)
ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
LOPERAMIDE HYDROCHLORIDE
loperamide hcl suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-0645 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 1 mg in 7.5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color GREEN (opaque, viscous) Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-0645-26 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/18/2012 2 NDC:0363-0645-34 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/18/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091292 05/18/2012 Labeler - Walgreen Company (008965063)