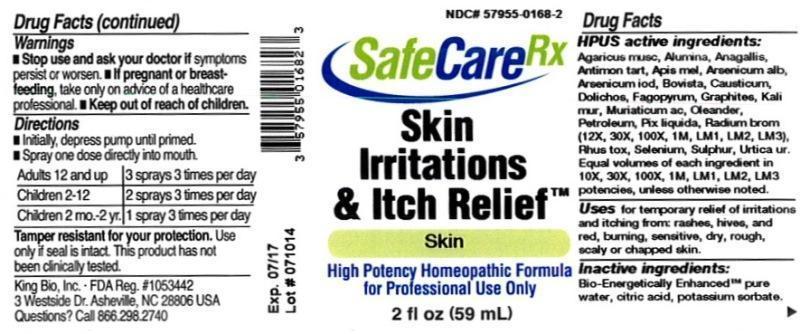
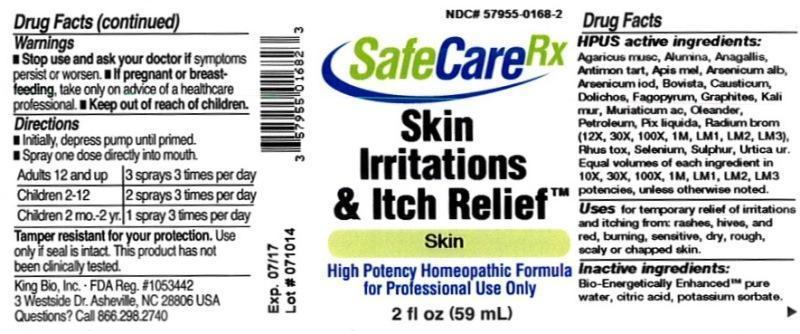
Label: SKIN IRRITATIONS AND ITCH RELIEF- arsenicum album, agaricus muscarius, alumina, anagallis arvensis, antimonium tartaricum, apis mellifica, arsenicum lodatum, bovista, causticum, dolichos pruriens, fagopyrum esculentum, graphites, kali muriaticum, muriaticum acidum, oleander, petroleum, pix liquida, radium bromatum, rhus toxicodendron, selenium metallicum, sulphur, urtica urens liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-0168-2 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 27, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Drug Facts__________________________________________________________________________________________________________
HPUS active ingredients: Arsenicum album, Agaricus muscarius, Alumina, Anagallis arvensis, Antimonium tartaricum, Apis mellifica, Arsenicum lodatum, Bovista, Causticum, Dolichos pruriens, Fagopyrum esculentum, Graphites, Kali muriaticum, Muriaticum acidum, Oleander, Petroleum, Pix liquida, Radium bromatum (12X, 30X,100X,1M,LM1,LM2, LM3), Rhus toxicodendron, Selenium metallicum, Sulphur, Urtica urens. Equal volumes of each ingredient in 10X, 30X, 100X, 1M, LM1, LM2, LM3 potencies, unless otherwise noted.
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKIN IRRITATIONS AND ITCH RELIEF
arsenicum album, agaricus muscarius, alumina, anagallis arvensis, antimonium tartaricum, apis mellifica, arsenicum lodatum, bovista, causticum, dolichos pruriens, fagopyrum esculentum, graphites, kali muriaticum, muriaticum acidum, oleander, petroleum, pix liquida, radium bromatum, rhus toxicodendron, selenium metallicum, sulphur, urtica urens liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-0168 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 10 [hp_X] in 59 mL AMANITA MUSCARIA FRUITING BODY (UNII: DIF093I037) (AMANITA MUSCARIA FRUITING BODY - UNII:DIF093I037) AMANITA MUSCARIA FRUITING BODY 10 [hp_X] in 59 mL ALUMINUM OXIDE (UNII: LMI26O6933) (ALUMINUM OXIDE - UNII:LMI26O6933) ALUMINUM OXIDE 10 [hp_X] in 59 mL ANAGALLIS ARVENSIS (UNII: 46883LR90E) (ANAGALLIS ARVENSIS - UNII:46883LR90E) ANAGALLIS ARVENSIS 10 [hp_X] in 59 mL ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 10 [hp_X] in 59 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 10 [hp_X] in 59 mL ARSENIC TRIIODIDE (UNII: 3029988O2T) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIIODIDE 10 [hp_X] in 59 mL LYCOPERDON UTRIFORME FRUITING BODY (UNII: K2A74U428F) (LYCOPERDON UTRIFORME FRUITING BODY - UNII:K2A74U428F) LYCOPERDON UTRIFORME FRUITING BODY 10 [hp_X] in 59 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 10 [hp_X] in 59 mL MUCUNA PRURIENS FRUIT TRICHOME (UNII: 3E271BSI0C) (MUCUNA PRURIENS FRUIT TRICHOME - UNII:3E271BSI0C) MUCUNA PRURIENS FRUIT TRICHOME 10 [hp_X] in 59 mL FAGOPYRUM ESCULENTUM (UNII: B10M69172N) (FAGOPYRUM ESCULENTUM - UNII:B10M69172N) FAGOPYRUM ESCULENTUM 10 [hp_X] in 59 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 10 [hp_X] in 59 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CATION 10 [hp_X] in 59 mL HYDROCHLORIC ACID (UNII: QTT17582CB) (HYDROCHLORIC ACID - UNII:QTT17582CB) HYDROCHLORIC ACID 10 [hp_X] in 59 mL NERIUM OLEANDER LEAF (UNII: 7KV510R6H6) (NERIUM OLEANDER LEAF - UNII:7KV510R6H6) NERIUM OLEANDER LEAF 10 [hp_X] in 59 mL KEROSENE (UNII: 1C89KKC04E) (KEROSENE - UNII:1C89KKC04E) KEROSENE 10 [hp_X] in 59 mL PINE TAR (UNII: YFH4WC535J) (PINE TAR - UNII:YFH4WC535J) PINE TAR 10 [hp_X] in 59 mL RADIUM BROMIDE (UNII: R74O7T8569) (RADIUM CATION - UNII:05456MVL7T) RADIUM BROMIDE 12 [hp_X] in 59 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 10 [hp_X] in 59 mL SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 10 [hp_X] in 59 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 10 [hp_X] in 59 mL URTICA URENS (UNII: IHN2NQ5OF9) (URTICA URENS - UNII:IHN2NQ5OF9) URTICA URENS 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-0168-2 59 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/06/2012 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture(57955-0168)