Label: CYCLOSPORINE capsule, liquid filled
- NDC Code(s): 75907-047-30, 75907-047-81, 75907-048-30, 75907-048-81
- Packager: Dr. Reddy's Laboratories Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING
Only physicians experienced in management of systemic immunosuppressive therapy for the indicated disease should prescribe cyclosporine [MODIFIED]. At doses used in solid organ transplantation, only physicians experienced in immunosuppressive therapy and management of organ transplant recipients should prescribe cyclosporine [MODIFIED]. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient.
Cyclosporine [MODIFIED], a systemic immunosuppressant, may increase the susceptibility to infection and the development of neoplasia. In kidney, liver, and heart transplant patients, cyclosporine [MODIFIED] may be administered with other immunosuppressive agents. Increased susceptibility to infection and the possible development of lymphoma and other neoplasms may result from the increase in the degree of immunosuppression in transplant patients.
Cyclosporine capsules [MODIFIED] and cyclosporine oral solution [MODIFIED] have increased bioavailability in comparison to Sandimmune®1 soft gelatin capsules (cyclosporine capsules) and Sandimmune® oral solution (cyclosporine oral solution). Cyclosporine [MODIFIED] and Sandimmune® are not bioequivalent and cannot be used interchangeably without physician supervision. For a given trough concentration, cyclosporine exposure will be greater with cyclosporine [MODIFIED] than with Sandimmune®. If a patient who is receiving exceptionally high doses of Sandimmune® is converted to cyclosporine [MODIFIED], particular caution should be exercised. Cyclosporine blood concentrations should be monitored in transplant and rheumatoid arthritis patients taking cyclosporine [MODIFIED] to avoid toxicity due to high concentrations. Dose adjustments should be made in transplant patients to minimize possible organ rejection due to low concentrations. Comparison of blood concentrations in the published literature with blood concentrations obtained using current assays must be done with detailed knowledge of the assay methods employed.(see DOSAGE AND ADMINISTRATION).
- 1
- Sandimmune® is a registered trademark of Novartis Pharmaceuticals Corporation.
-
BOXED WARNING
(What is this?)
For Psoriasis Patients
(See also Boxed WARNINGS above)
Psoriasis patients previously treated with PUVA and to a lesser extent, methotrexate or other immunosuppressive agents, UVB, coal tar, or radiation therapy, are at an increased risk of developing skin malignancies when taking cyclosporine [MODIFIED].
Cyclosporine, the active ingredient in cyclosporine capsules [MODIFIED] and cyclosporine oral solution [MODIFIED], in recommended dosages, can cause systemic hypertension and nephrotoxicity. The risk increases with increasing dose and duration of cyclosporine therapy. Renal dysfunction, including structural kidney damage, is a potential consequence of cyclosporine, and therefore, renal function must be monitored during therapy.
-
DESCRIPTION
Cyclosporine capsules, USP [MODIFIED] and cyclosporine oral solution, USP [MODIFIED] are oral formulations of cyclosporine that immediately forms a microemulsion in an aqueous environment.
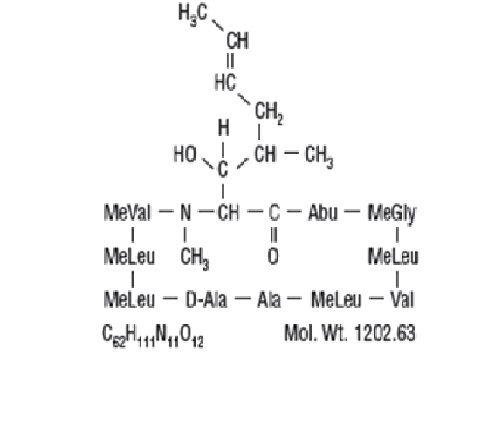
Cyclosporine, the active principle in cyclosporine capsules [MODIFIED] and cyclosporine oral solution, USP [MODIFIED], is a cyclic polypeptide immunosuppressant agent consisting of 11 amino acids. It is produced as a metabolite by the fungus species Beauveria nivea.
Chemically, cyclosporine is designated as [R-[R*,R*-(E)]]-cyclic-(L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-3-hydroxy-N, 4-dimethyl-L-2-amino-6-octenoyl-L-α-amino-butyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl).
Each soft gelatin cyclosporine capsule, USP [Modified] for oral administration contains 25 mg or 100 mg of cyclosporine, USP. In addition, each capsule contains the following inactive ingredients: Caprylic/capric triglyceride, dl-alpha-tocopherol, gelatin, glycerin, glyceryl caprylate, PEG-8 caprylic/capric glycerides, PEG-35 castor oil, red iron oxide, shellac, sorbitol special 76% and titanium dioxide USP.
Cyclosporine oral solution, USP [MODIFIED] contains 100 mg/mL of cyclosporine, USP. Inactive ingredients: Caprylic/capric triglyceride, dl-alpha-tocopherol, glyceryl caprylate, PEG-8 caprylic/capric glycerides, PEG-35 castor oil.
The chemical structure of cyclosporine, USP (also known as cyclosporin A) is:
-
CLINICAL PHARMACOLOGY
Cyclosporine is a potent immunosuppressive agent that in animals prolongs survival of allogeneic transplants involving skin, kidney, liver, heart, pancreas, bone marrow, small intestine, and lung. Cyclosporine has been demonstrated to suppress some humoral immunity and to a greater extent, cell-mediated immune reactions such as allograft rejection, delayed hypersensitivity, experimental allergic encephalomyelitis, Freund's adjuvant arthritis, and graft vs. host disease in many animal species for a variety of organs.
The effectiveness of cyclosporine results from specific and reversible inhibition of immunocompetent lymphocytes in the G0- and G1-phase of the cell cycle. T-lymphocytes are preferentially inhibited. The T-helper cell is the main target, although the T-suppressor cell may also be suppressed. Cyclosporine also inhibits lymphokine production and release including interleukin-2.
No effects on phagocytic function (changes in enzyme secretions, chemotactic migration of granulocytes, macrophage migration, carbon clearance in vivo) have been detected in animals. Cyclosporine does not cause bone marrow suppression in animal models or man.
Pharmacokinetics
The immunosuppressive activity of cyclosporine is primarily due to parent drug. Following oral administration, absorption of cyclosporine is incomplete. The extent of absorption of cyclosporine is dependent on the individual patient, the patient population, and the formulation. Elimination of cyclosporine is primarily biliary with only 6% of the dose (parent drug and metabolites) excreted in urine. The disposition of cyclosporine from blood is generally biphasic, with a terminal half-life of approximately 8.4 hours (range 5 to 18 hours). Following intravenous administration, the blood clearance of cyclosporine (assay: HPLC) is approximately 5 to 7 mL/min/kg in adult recipients of renal or liver allografts. Blood cyclosporine clearance appears to be slightly slower in cardiac transplant patients.
Cyclosporine capsules [MODIFIED] and cyclosporine oral solution [MODIFIED] are bioequivalent. Cyclosporine oral solution [MODIFIED] diluted with orange juice or apple juice is bioequivalent to cyclosporine oral solution [MODIFIED] diluted with water. The effect of milk on the bioavailability of cyclosporine when administered as cyclosporine oral solution [MODIFIED] has not been evaluated.
The relationship between administered dose and exposure (area under the concentration versus time curve, AUC) is linear within the therapeutic dose range. The intersubject variability (total, %CV) of cyclosporine exposure (AUC) when cyclosporine [MODIFIED] or Sandimmune® is administered ranges from approximately 20% to 50% in renal transplant patients. This intersubject variability contributes to the need for individualization of the dosing regimen for optimal therapy (see DOSAGE AND ADMINISTRATION). Intrasubject variability of AUC in renal transplant recipients (%CV) was 9% to 21% for cyclosporine [MODIFIED] and 19% to 26% for Sandimmune®. In the same studies, intrasubject variability of trough concentrations (%CV) was 17% to 30% for cyclosporine [MODIFIED] and 16% to 38% for Sandimmune®.
Absorption
Cyclosporine [MODIFIED] has increased bioavailability compared to Sandimmune® (cyclosporine). The absolute bioavailability of cyclosporine administered as Sandimmune® is dependent on the patient population, estimated to be less than 10% in liver transplant patients and as great as 89% in some renal transplant patients. The absolute bioavailability of cyclosporine administered as cyclosporine [MODIFIED] has not been determined in adults. In studies of renal transplant, rheumatoid arthritis and psoriasis patients, the mean cyclosporine AUC was approximately 20% to 50% greater and the peak blood cyclosporine concentration (Cmax) was approximately 40% to 106% greater following administration of cyclosporine [MODIFIED] compared to following administration of Sandimmune®. The dose normalized AUC in de novo liver transplant patients administered cyclosporine [MODIFIED] 28 days after transplantation was 50% greater and Cmax was 90% greater than in those patients administered Sandimmune®. AUC and Cmax are also increased (cyclosporine [MODIFIED] relative to Sandimmune®) in heart transplant patients, but data are very limited. Although the AUC and Cmax values are higher on cyclosporine [MODIFIED] relative to Sandimmune®, the pre-dose trough concentrations (dose-normalized) are similar for the two formulations.
Following oral administration of cyclosporine [MODIFIED], the time to peak blood cyclosporine concentrations (Tmax) ranged from 1.5 to 2 hours. The administration of food with cyclosporine [MODIFIED] decreases the cyclosporine AUC and Cmax. A high fat meal (669 kcal, 45 grams fat) consumed within one-half hour before cyclosporine [MODIFIED] administration decreased the AUC by 13% and Cmax by 33%. The effects of a low fat meal (667 kcal, 15 grams fat) were similar.
The effect of T-tube diversion of bile on the absorption of cyclosporine from cyclosporine [MODIFIED] was investigated in eleven de novo liver transplant patients. When the patients were administered cyclosporine [MODIFIED] with and without T-tube diversion of bile, very little difference in absorption was observed, as measured by the change in maximal cyclosporine blood concentrations from pre-dose values with the T-tube closed relative to when it was open: 6.9±41% (range: 55% to 68%).
Pharmacokinetic Parameters (mean ± SD) Patient Population Dose/day*
(mg/d)Dose/Weight
(mg/kg/d)AUC†
(ng∙mL)Cmax
(ng/mL)Trough‡
(ng/mL)CL/F
(mL/min)CL/F
(mL/min/kg)- *
- Total daily dose was divided into two doses administered every 12 hours
- †
- AUC was measured over one dosing interval
- ‡
- Trough concentration was measured just prior to the morning cyclosporine [Modified] dose, approximately 12 hours after the previous dose
- §
- Assay: TDx specific monoclonal fluorescence polarization immunoassay
- ¶
- #
- Assay: Cyclo-trac specific monoclonal radioimmunoassay
- Þ
- Assay: INCSTAR specific monoclonal radioimmunoassay
De novo renal transplant§
Week 4
(N=37)
597±174 7.95±2.81 8772±2089 1802±428 361±129 593±204 7.8±2.9 Stable renal transplant ¶
(N=55)344±122 4.10±1.58 6035±2194 1333±469 251±116 492±140 5.9±2.1 De novo liver transplant#
Week 4
(N=18)458±190 6.89±3.68 7187±2816 1555±740 268±101 577±309 8.6±5.7 De novo rheumatoid arthritisÞ
(N=23)182±55.6 2.37±0.36 2641±877 728±263 96.4±37.7 613±196 8.3±2.8 De novo psoriasis
Week 4
(N=18)189±69.8 2.48±0.65 2324±1048 655±186 74.9±46.7 723±186 10.2±3.9 Distribution
Cyclosporine is distributed largely outside the blood volume. The steady state volume of distribution during intravenous dosing has been reported as 3 to 5 L/kg in solid organ transplant recipients. In blood, the distribution is concentration dependent. Approximately 33% to 47% is in plasma, 4% to 9% in lymphocytes, 5% to 12% in granulocytes, and 41% to 58% in erythrocytes. At high concentrations, the binding capacity of leukocytes and erythrocytes becomes saturated. In plasma, approximately 90% is bound to proteins, primarily lipoproteins. Cyclosporine is excreted in human milk. (See PRECAUTIONS, Nursing Mothers)
Metabolism
Cyclosporine is extensively metabolized by the cytochrome P-450 3A enzyme system in the liver, and to a lesser degree in the gastrointestinal tract, and the kidney. The metabolism of cyclosporine can be altered by the coadministration of a variety of agents. (See PRECAUTIONS, Drug Interactions) At least 25 metabolites have been identified from human bile, feces, blood, and urine. The biological activity of the metabolites and their contributions to toxicity are considerably less than those of the parent compound. The major metabolites (M1, M9, and M4N) result from oxidation at the 1-beta, 9-gamma, and 4-N-demethylated positions, respectively. At steady state following the oral administration of Sandimmune®, the mean AUCs for blood concentrations of M1, M9, and M4N are about 70%, 21%, and 7.5% of the AUC for blood cyclosporine concentrations, respectively. Based on blood concentration data from stable renal transplant patients (13 patients administered cyclosporine [MODIFIED] and Sandimmune® in a crossover study), and bile concentration data from de novo liver transplant patients (4 administered cyclosporine [MODIFIED], 3 administered Sandimmune®, the percentage of dose present as M1, M9, and M4N metabolites is similar when either cyclosporine [MODIFIED] or Sandimmune® is administered.
Excretion
Only 0.1% of a cyclosporine dose is excreted unchanged in the urine. Elimination is primarily biliary with only 6% of the dose (parent drug and metabolites) excreted in the urine. Neither dialysis nor renal failure alter cyclosporine clearance significantly.
Drug Interactions
(See PRECAUTIONS, Drug Interactions) When diclofenac or methotrexate was coadministered with cyclosporine in rheumatoid arthritis patients, the AUC of diclofenac and methotrexate, each was significantly increased. (See PRECAUTIONS, Drug Interactions) No clinically significant pharmacokinetic interactions occurred between cyclosporine and aspirin, ketoprofen, piroxicam, or indomethacin.
Specific Populations
Renal impairment
In a study performed in 4 subjects with end-stage renal disease (creatinine clearance < 5 mL/min), an intravenous infusion of 3.5 mg/kg of cyclosporine over 4 hours administered at the end of a hemodialysis session resulted in a mean volume of distribution (Vdss) of 3.49 L/kg and systemic clearance (CL) of 0.369 L/hr/kg. This systemic CL (0.369 L/hr/kg) was approximately two thirds of the mean systemic CL (0.56 L/hr/kg) of cyclosporine in historical control subjects with normal renal function. In 5 liver transplant patients, the mean clearance of cyclosporine on and off hemodialysis was 463 mL/min and 398 mL/min, respectively. Less than 1% of the dose of cyclosporine was recovered in the dialysate.
Hepatic Impairment
Cyclosporine is extensively metabolized by the liver. Since severe hepatic impairment may result in significantly increased cyclosporine exposures, the dosage of cyclosporine may need to be reduced in these patients.
Pediatric Population
Pharmacokinetic data from pediatric patients administered cyclosporine [MODIFIED] or Sandimmune® are very limited. In 15 renal transplant patients aged 3 to 16 years, cyclosporine whole blood clearance after IV administration of Sandimmune® was 10.6±3.7 mL/min/kg (assay: Cyclo-trac specific RIA). In a study of 7 renal transplant patients aged 2 to 16, the cyclosporine clearance ranged from 9.8 to 15.5 mL/min/kg. In 9 liver transplant patients aged 0.6 to 5.6 years, clearance was 9.3±5.4 mL/min/kg (assay: HPLC).
In the pediatric population, cyclosporine [MODIFIED] also demonstrates an increased bioavailability as compared to Sandimmune®. In 7 liver de novo transplant patients aged 1.4 to 10 years, the absolute bioavailability of cyclosporine [MODIFIED] was 43% (range: 30% to 68%) and for Sandimmune® in the same individuals absolute bioavailability was 28% (range: 17% to 42%).
Pediatric Pharmacokinetic Parameters (mean±SD) Patient Population Dose/day
(mg/d)Dose/weight
(mg/kg/d)AUC*
(ng∙hr/mL)Cmax
(ng/mL)CL/F
(mL/min)CL/F
(mL/min/kg)Stable liver transplant† Age 2 to 8, Dosed TID (N=9) 101±25 5.95±1.32 2163±801 629±219 285±94 16.6±4.3 Age 8 to 15, Dosed BID (N=8) 188±55 4.96±2.09 4272±1462 975±281 378±80 10.2±4 Stable liver transplant‡ Age 3, Dosed BID (N=1) 120 8.33 5832 1050 171 11.9 Age 8 to 15, Dosed BID (N=5) 158±55 5.51±1.91 4452±2475 1013±635 328±121 11±1.9 Stable renal transplant Age 7 to 15, Dosed BID (N=5) 328±83 7.37±4.11 6922±1988 1827±487 418±143 8.7±2.9 Geriatric Population
Comparison of single dose data from both normal elderly volunteers (N=18, mean age 69 years) and elderly rheumatoid arthritis patients (N=16, mean age 68 years) to single dose data in young adult volunteers (N=16, mean age 26 years) showed no significant difference in the pharmacokinetic parameters.
CLINICAL TRIALS
Rheumatoid Arthritis
The effectiveness of Sandimmune® (cyclosporine) and cyclosporine [MODIFIED] in the treatment of severe rheumatoid arthritis was evaluated in 5 clinical studies involving a total of 728 cyclosporine treated patients and 273 placebo treated patients.
A summary of the results is presented for the "responder" rates per treatment group, with a responder being defined as a patient having completed the trial with a 20% improvement in the tender and the swollen joint count and a 20% improvement in 2 of 4 of investigator global, patient global, disability, and erythrocyte sedimentation rates (ESR) for the Studies 651 and 652 and 3 of 5 of investigator global, patient global, disability, visual analog pain, and ESR for Studies 2008, 654 and 302.
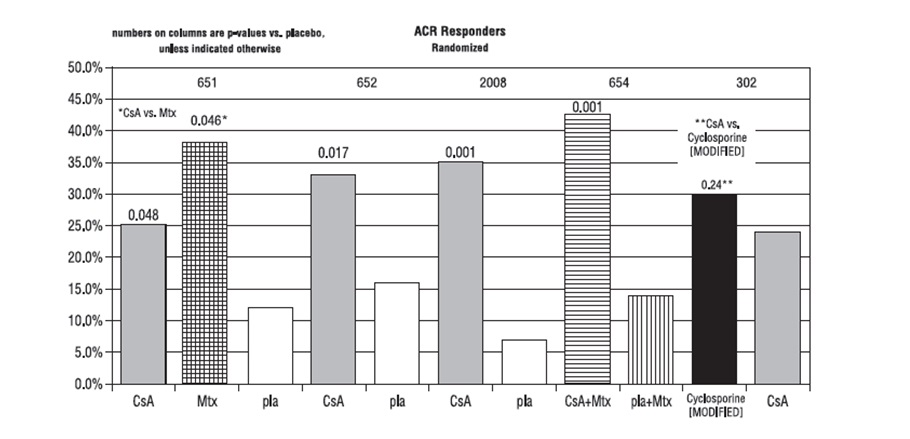
Study 651 enrolled 264 patients with active rheumatoid arthritis with at least 20 involved joints, who had failed at least one major RA drug, using a 3:3:2 randomization to one of the following three groups: (1) cyclosporine dosed at 2.5 to 5 mg/kg/day, (2) methotrexate at 7.5 to 15 mg/week, or (3) placebo. Treatment duration was 24 weeks. The mean cyclosporine dose at the last visit was 3.1 mg/kg/day. See Graph below.
Study 652 enrolled 250 patients with active RA with >6 active painful or tender joints who had failed at least one major RA drug. Patients were randomized using a 3:3:2 randomization to 1 of 3 treatment arms: (1) 1.5 to 5 mg/kg/day of cyclosporine, (2) 2.5 to 5 mg/kg/day of cyclosporine, and (3) placebo. Treatment duration was 16 weeks. The mean cyclosporine dose for group 2 at the last visit was 2.92 mg/kg/day. See Graph below.
Study 2008 enrolled 144 patients with active RA and >6 active joints who had unsuccessful treatment courses of aspirin and gold or Penicillamine. Patients were randomized to 1 of 2 treatment groups: (1) cyclosporine 2.5 to 5 mg/kg/day with adjustments after the first month to achieve a target trough level and (2) placebo. Treatment duration was 24 weeks. The mean cyclosporine dose at the last visit was 3.63 mg/kg/day. See Graph below.
Study 654 enrolled 148 patients who remained with active joint counts of 6 or more despite treatment with maximally tolerated methotrexate doses for at least three months. Patients continued to take their current dose of methotrexate and were randomized to receive, in addition, one of the following medications: (1) cyclosporine 2.5 mg/kg/day with dose increases of 0.5 mg/kg/day at weeks 2 and 4 if there was no evidence of toxicity and further increases of 0.5 mg/kg/day at weeks 8 and 16 if a <30% decrease in active joint count occurred without any significant toxicity; dose decreases could be made at any time for toxicity or (2) placebo. Treatment duration was 24 weeks. The mean cyclosporine dose at the last visit was 2.8 mg/kg/day (range: 1.3 to 4.1). See Graph below.
Study 302 enrolled 299 patients with severe active RA, 99% of whom were unresponsive or intolerant to at least one prior major RA drug. Patients were randomized to 1 of 2 treatment groups: (1) cyclosporine [MODIFIED] and (2) Sandimmune®, both of which were started at 2.5 mg/kg/day and increased after 4 weeks for inefficacy in increments of 0.5 mg/kg/day to a maximum of 5 mg/kg/day and decreased at any time for toxicity. Treatment duration was 24 weeks. The mean cyclosporine dose at the last visit was 2.91 mg/kg/day (range: 0.72 to 5.17) for cyclosporine [MODIFIED] and 3.27 mg/kg/day (range: 0.73 to 5.68) for Sandimmune®. See Graph below.
-
INDICATIONS AND USAGE
Kidney, Liver, and Heart Transplantation
Cyclosporine capsules, USP [MODIFIED] and cyclosporine oral solution, USP [MODIFIED] are indicated for the prophylaxis of organ rejection in kidney, liver, and heart allogeneic transplants. Cyclosporine capsules, USP [MODIFIED] and cyclosporine oral solution, USP [MODIFIED] have been used in combination with azathioprine and corticosteroids.
Rheumatoid Arthritis
Cyclosporine capsules, USP [MODIFIED] and cyclosporine oral solution, USP [MODIFIED] are indicated for the treatment of patients with severe active, rheumatoid arthritis where the disease has not adequately responded to methotrexate. Cyclosporine capsules, USP [MODIFIED] and cyclosporine oral solution, USP [MODIFIED] can be used in combination with methotrexate in rheumatoid arthritis patients who do not respond adequately to methotrexate alone.
Psoriasis
Cyclosporine capsules, USP [MODIFIED] and cyclosporine oral solution, USP [MODIFIED] are indicated for the treatment of adult, nonimmunocompromised patients with severe (i.e., extensive and/or disabling), recalcitrant, plaque psoriasis who have failed to respond to at least one systemic therapy (e.g., PUVA, retinoids, or methotrexate) or in patients for whom other systemic therapies are contraindicated, or cannot be tolerated.
While rebound rarely occurs, most patients will experience relapse with cyclosporine, USP [MODIFIED] as with other therapies upon cessation of treatment.
-
CONTRAINDICATIONS
General
Cyclosporine [MODIFIED] is contraindicated in patients with a hypersensitivity to cyclosporine or to any of the ingredients of the formulation.
Rheumatoid Arthritis
Rheumatoid arthritis patients with abnormal renal function, uncontrolled hypertension, or malignancies should not receive cyclosporine [MODIFIED].
Psoriasis
Psoriasis patients who are treated with cyclosporine [MODIFIED] should not receive concomitant PUVA or UVB therapy, methotrexate or other immunosuppressive agents, coal tar or radiation therapy. Psoriasis patients with abnormal renal function, uncontrolled hypertension, or malignancies should not receive cyclosporine [MODIFIED].
-
WARNINGS
(See also Boxed WARNING)
All Patients
Cyclosporine, the active ingredient in cyclosporine capsules [MODIFIED] and cyclosporine oral solution [MODIFIED], can cause nephrotoxicity and hepatotoxicity. The risk increases with increasing doses of cyclosporine. Renal dysfunction including structural kidney damage is a potential consequence of cyclosporine [MODIFIED] and therefore renal function must be monitored during therapy. Care should be taken in using cyclosporine with nephrotoxic drugs. (See PRECAUTIONS)
Patients receiving cyclosporine [MODIFIED] require frequent monitoring of serum creatinine. (See Special Monitoring under DOSAGE AND ADMINISTRATION) Elderly patients should be monitored with particular care, since decreases in renal function also occur with age. If patients are not properly monitored and doses are not properly adjusted, cyclosporine therapy can be associated with the occurrence of structural kidney damage and persistent renal dysfunction.
An increase in serum creatinine and BUN may occur during cyclosporine [MODIFIED] therapy and reflect a reduction in the glomerular filtration rate. Impaired renal function at any time requires close monitoring, and frequent dosage adjustment may be indicated. The frequency and severity of serum creatinine elevations increase with dose and duration of cyclosporine therapy. These elevations are likely to become more pronounced without dose reduction or discontinuation.
Because cyclosporine capsules [MODIFIED] and cyclosporine oral solution [MODIFIED] are not bioequivalent to Sandimmune® (cyclosporine), conversion from cyclosporine capsules [MODIFIED] and cyclosporine oral solution [MODIFIED] to Sandimmune® using a 1:1 ratio (mg/kg/day) may result in lower cyclosporine blood concentrations. Conversion from cyclosporine [MODIFIED] to Sandimmune® should be made with increased monitoring to avoid the potential of underdosing.
Kidney, Liver, and Heart Transplant
Nephrotoxicity
Cyclosporine, the active ingredient of cyclosporine [MODIFIED], can cause nephrotoxicity and hepatotoxicity when used in high doses. It is not unusual for serum creatinine and BUN levels to be elevated during cyclosporine therapy. These elevations in renal transplant patients do not necessarily indicate rejection, and each patient must be fully evaluated before dosage adjustment is initiated.
Based on the historical Sandimmune® experience with oral solution, nephrotoxicity associated with cyclosporine had been noted in 25% of cases of renal transplantation, 38% of cases of cardiac transplantation, and 37% of cases of liver transplantation. Mild nephrotoxicity was generally noted 2 to 3 months after renal transplant and consisted of an arrest in the fall of the pre-operative elevations of BUN and creatinine at a range of 35 to 45 mg/dL and 2 to 2.5 mg/dL respectively. These elevations were often responsive to cyclosporine dosage reduction.
More overt nephrotoxicity was seen early after transplantation and was characterized by a rapidly rising BUN and creatinine. Since these events are similar to renal rejection episodes, care must be taken to differentiate between them. This form of nephrotoxicity is usually responsive to cyclosporine dosage reduction.
Although specific diagnostic criteria which reliably differentiate renal graft rejection from drug toxicity have not been found, a number of parameters have been significantly associated with one or the other. It should be noted, however, that up to 20% of patients may have simultaneous nephrotoxicity and rejection.
Nephrotoxicity vs. Rejection Parameter Nephrotoxicity Rejection History Donor >50 years old or hypotensive
Prolonged kidney preservation
Prolonged anastomosis time
Concomitant nephrotoxic drugsAnti-donor immune response
Retransplant patientClinical Often >6 weeks postop*
Prolonged initial nonfunction
(acute tubular necrosis)Often <4 weeks postop
Fever >37.5°C
Weight gain >0.5 kg
Graft swelling and tenderness
Decrease in daily urine volume >500 mL (or 50%)Laboratory CyA serum trough level >200 ng/mL
Gradual rise in Cr (<0.15 mg/dL/day)†
Cr plateau <25% above baseline
BUN/Cr ≥20CyA serum trough level <150 ng/mL
Rapid rise in Cr (>0.3 mg/dL/day)
Cr >25% above baseline
BUN/Cr <20Biopsy Arteriolopathy (medial hypertrophy, hyalinosis, nodular deposits, intimal thickening, endothelial vacuolization, vacuolization, progressive scarring) Endovasculitis (proliferation, intimalarteritis, necrosis, sclerosis) Tubular atrophy, isometric vacuolization, isolated calcifications
Minimal edema
Mild focal infiltrates‡Tubulitis with RBC and WBC casts, some irregular vacuolization
Interstitial edema and hemorrhage
Diffuse moderate to severe mononuclear infiltrates§ Glomerulitis (mononuclear cells)Diffuse interstitial fibrosis, often striped form Aspiration Cytology CyA deposits in tubular and endothelial cells
Fine isometric vacuolization of tubular cellsInflammatory infiltrate with mononuclear phagocytes, macrophages, lymphoblastoid cells, and activated T-cells
These strongly express HLA-DR antigensUrine Cytology Tubular cells with vacuolization and granularization Degenerative tubular cells, plasma cells, and lymphocyturia >20% of sediment Manometry Intracapsular pressure <40 mm Hg Intracapsular pressure >40 mm Hg Ultrasonography Unchanged graft cross sectional area Increase in graft cross sectional area
AP diameter ≥ Transverse diameterMagnetic Resonance Imagery Normal appearance Loss of distinct corticomedullary junction, swelling image intensity of parachyma approaching that of psoas, loss of hilar fat Radionuclide Scan Normal or generally decreased perfusion
Decrease in tubular function
(131 I-hippuran) > decrease in perfusion (99m Tc DTPA)Patchy arterial flow
Decrease in perfusion > decrease in tubular function
Increased uptake of Indium 111 labeled platelets or Tc-99m in colloidTherapy Responds to decreased cyclosporine Responds to increased steroids or antilymphocyte globulin A form of a cyclosporine-associated nephropathy is characterized by serial deterioration in renal function and morphologic changes in the kidneys. From 5% to 15% of transplant recipients who have received cyclosporine will fail to show a reduction in rising serum creatinine despite a decrease or discontinuation of cyclosporine therapy. Renal biopsies from these patients will demonstrate one or several of the following alterations: tubular vacuolization, tubular microcalcifications, peritubular capillary congestion, arteriolopathy, and a striped form of interstitial fibrosis with tubular atrophy. Though none of these morphologic changes is entirely specific, a diagnosis of cyclosporine-associated structural nephrotoxicity requires evidence of these findings.
When considering the development of cyclosporine-associated nephropathy, it is noteworthy that several authors have reported an association between the appearance of interstitial fibrosis and higher cumulative doses or persistently high circulating trough concentrations of cyclosporine. This is particularly true during the first 6 post-transplant months when the dosage tends to be highest and when, in kidney recipients, the organ appears to be most vulnerable to the toxic effects of cyclosporine. Among other contributing factors to the development of interstitial fibrosis in these patients are prolonged perfusion time, warm ischemia time, as well as episodes of acute toxicity, and acute and chronic rejection. The reversibility of interstitial fibrosis and its correlation to renal function have not yet been determined. Reversibility of arteriolopathy has been reported after stopping cyclosporine or lowering the dosage.
Impaired renal function at any time requires close monitoring, and frequent dosage adjustment may be indicated.
In the event of severe and unremitting rejection, when rescue therapy with pulse steroids and monoclonal antibodies fail to reverse the rejection episode, it may be preferable to switch to alternative immunosuppressive therapy rather than increase the cyclosporine [MODIFIED] dose to excessive blood concentrations.
Due to the potential for additive or synergistic impairment of renal function, caution should be exercised when coadministering cyclosporine with other drugs that may impair renal function. (See PRECAUTIONS, Drug Interactions)
Thrombotic Microangiopathy
Occasionally patients have developed a syndrome of thrombocytopenia and microangiopathic hemolytic anemia which may result in graft failure. The vasculopathy can occur in the absence of rejection and is accompanied by avid platelet consumption within the graft as demonstrated by Indium 111 labeled platelet studies. Neither the pathogenesis nor the management of this syndrome is clear. Though resolution has occurred after reduction or discontinuation of cyclosporine and 1) administration of streptokinase and heparin or 2) plasmapheresis, this appears to depend upon early detection with Indium 111 labeled platelet scans. (See ADVERSE REACTIONS)
Hyperkalemia
Significant hyperkalemia (sometimes associated with hyperchloremic metabolic acidosis) and hyperuricemia have been seen occasionally in individual patients.
Hepatotoxicity
Cases of hepatotoxicity and liver injury including cholestasis, jaundice, hepatitis and liver failure have been reported in patients treated with cyclosporine. Most reports included patients with significant co-morbidities, underlying conditions and other confounding factors including infectious complications and comedications with hepatotoxic potential. In some cases, mainly in transplant patients, fatal outcomes have been reported (see ADVERSE REACTIONS, Postmarketing Experience, Kidney, Liver and Heart Transplantation).
Hepatotoxicity usually manifested by elevations of hepatic enzymes and bilirubin, was reported in patients treated with cyclosporine in clinical trials: 4% in renal transplantation, 7% in cardiac transplantation, and 4% of cases of in liver transplantation. This was usually noted during the first month of therapy when high doses of cyclosporine were used. The chemistry elevations usually decreased with a reduction in dosage.
Malignancies
As in patients receiving other immunosuppressants, those patients receiving cyclosporine are at increased risk for development of lymphomas and other malignancies, particularly those of the skin. Patients taking cyclosporine should be warned to avoid excess ultraviolet light exposure. The increased risk appears related to the intensity and duration of immunosuppression rather than to the use of specific agents. Because of the danger of oversuppression of the immune system resulting in increased risk of infection or malignancy, a treatment regimen containing multiple immunosuppressants should be used with caution. Transplant patients receiving cyclosporine are at increased risk for serious infection with fatal outcome.
Serious Infections
Patients receiving immunosuppressants, including cyclosporine [MODIFIED], are at increased risk of developing bacterial, viral, fungal, and protozoal infections, including opportunistic infections. These infections may lead to serious, including fatal, outcomes (see BOXED WARNING and ADVERSE REACTIONS).
Polyoma Virus Infections
Patients receiving immunosuppressants including cyclosporine are at increased risk for opportunistic infections, including polyoma virus infections. Polyoma virus infections in transplant patients may have serious, and sometimes, fatal outcomes. These include cases of JC virus-associated progressive multifocal leukoencephalopathy (PML) and polyoma virus-associated nephropathy (PVAN) especially due to BK virus infection which have been observed in patients receiving cyclosporine.
PVAN is associated with serious outcomes, including deteriorating renal function and renal graft loss, (see ADVERSE REACTIONS/Postmarketing Experience, Kidney, Liver and Heart Transplantation). Patient monitoring may help detect patients at risk for PVAN.Cases of PML have been reported in patients treated with cyclosporine [MODIFIED]. PML, which is sometimes fatal, commonly presents with hemiparesis, apathy, confusion, cognitive deficiencies and ataxia. Risk factors for PML include treatment with immunosuppressant therapies and impairment of immune function. In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms and consultation with a neurologist should be considered as clinically indicated.
Consideration should be given to reducing the total immunosuppression in transplant patients who develop PML or PVAN. However, reduced immunosuppression may place the graft at risk.
Neurotoxicity
There have been reports of convulsions in adult and pediatric patients receiving cyclosporine, particularly in combination with high dose methylprednisolone.
Encephalopathy, including Posterior Reversible Encephalopathy Syndrome (PRES), has been described both in post-marketing reports and in the literature. Manifestations include impaired consciousness, convulsions, visual disturbances (including blindness), loss of motor function, movement disorders and psychiatric disturbances. In many cases, changes in the white matter have been detected using imaging techniques and pathologic specimens. Predisposing factors such as hypertension, hypomagnesemia, hypocholesterolemia, high-dose corticosteroids, high cyclosporine blood concentrations, and graft-versus-host disease have been noted in many but not all of the reported cases. The changes in most cases have been reversible upon discontinuation of cyclosporine, and in some cases improvement was noted after reduction of dose. It appears that patients receiving liver transplant are more susceptible to encephalopathy than those receiving kidney transplant. Another rare manifestation of cyclosporine-induced neurotoxicity, occurring in transplant patients more frequently than in other indications, is optic disc edema including papilloedema, with possible visual impairment, secondary to benign intracranial hypertension.
Care should be taken in using cyclosporine with nephrotoxic drugs. (See PRECAUTIONS)
Rheumatoid Arthritis
Cyclosporine nephropathy was detected in renal biopsies of 6 out of 60 (10%) rheumatoid arthritis patients after the average treatment duration of 19 months. Only one patient, out of these 6 patients, was treated with a dose < 4 mg/kg/day. Serum creatinine improved in all but one patient after discontinuation of cyclosporine. The "maximal creatinine increase" appears to be a factor in predicting cyclosporine nephropathy.
There is a potential, as with other immunosuppressive agents, for an increase in the occurrence of malignant lymphomas with cyclosporine. It is not clear whether the risk with cyclosporine is greater than that in rheumatoid arthritis patients or in rheumatoid arthritis patients on cytotoxic treatment for this indication. Five cases of lymphoma were detected: four in a survey of approximately 2,300 patients treated with cyclosporine for rheumatoid arthritis, and another case of lymphoma was reported in a clinical trial. Although other tumors (12 skin cancers, 24 solid tumors of diverse types, and 1 multiple myeloma) were also reported in this survey, epidemiologic analyses did not support a relationship to cyclosporine other than for malignant lymphomas.
Patients should be thoroughly evaluated before and during cyclosporine [MODIFIED] treatment for the development of malignancies. Moreover, use of cyclosporine [MODIFIED] therapy with other immunosuppressive agents may induce an excessive immunosuppression which is known to increase the risk of malignancy.
Psoriasis
(See also Boxed WARNINGS for Psoriasis) Since cyclosporine is a potent immunosuppressive agent with a number of potentially serious side effects, the risks and benefits of using cyclosporine [MODIFIED] should be considered before treatment of patients with psoriasis. Cyclosporine, the active ingredient in cyclosporine [MODIFIED], can cause nephrotoxicity and hypertension (see PRECAUTIONS) and the risk increases with increasing dose and duration of therapy. Patients who may be at increased risk such as those with abnormal renal function, uncontrolled hypertension or malignancies, should not receive cyclosporine [MODIFIED].
Renal dysfunction is a potential consequence of cyclosporine [MODIFIED], therefore renal function must be monitored during therapy.
Patients receiving cyclosporine [MODIFIED] require frequent monitoring of serum creatinine. (See Special Monitoring under DOSAGE AND ADMINISTRATION) Elderly patients should be monitored with particular care, since decreases in renal function also occur with age. If patients are not properly monitored and doses are not properly adjusted, cyclosporine therapy can cause structural kidney damage and persistent renal dysfunction.
An increase in serum creatinine and BUN may occur during cyclosporine [MODIFIED] therapy and reflects a reduction in the glomerular filtration rate.
Kidney biopsies from 86 psoriasis patients treated for a mean duration of 23 months with 1.2 to 7.6 mg/kg/day of cyclosporine showed evidence of cyclosporine nephropathy in 18/86 (21%) of the patients. The pathology consisted of renal tubular atrophy and interstitial fibrosis. On repeat biopsy of 13 of these patients maintained on various dosages of cyclosporine for a mean of 2 additional years, the number with cyclosporine induced nephropathy rose to 26/86 (30%). The majority of patients (19/26) were on a dose of >5 mg/kg/day (the highest recommended dose is 4 mg/kg/day). The patients were also on cyclosporine for greater than 15 months (18/26) and/or had a clinically significant increase in serum creatinine for greater than 1 month (21/26). Creatinine levels returned to normal range in 7 of 11 patients in whom cyclosporine therapy was discontinued.
There is an increased risk for the development of skin and lymphoproliferative malignancies in cyclosporine-treated psoriasis patients. The relative risk of malignancies is comparable to that observed in psoriasis patients treated with other immunosuppressive agents.
Tumors were reported in 32 (2.2%) of 1439 psoriasis patients treated with cyclosporine worldwide from clinical trials. Additional tumors have been reported in 7 patients in cyclosporine postmarketing experience. Skin malignancies were reported in 16 (1.1%) of these patients; all but 2 of them had previously received PUVA therapy. Methotrexate was received by 7 patients. UVB and coal tar had been used by 2 and 3 patients, respectively. Seven patients had either a history of previous skin cancer or a potentially predisposing lesion was present prior to cyclosporine exposure. Of the 16 patients with skin cancer, 11 patients had 18 squamous cell carcinomas and 7 patients had 10 basal cell carcinomas.
There were two lymphoproliferative malignancies: one case of non-Hodgkin's lymphoma which required chemotherapy, and one case of mycosis fungoides which regressed spontaneously upon discontinuation of cyclosporine. There were four cases of benign lymphocytic infiltration: 3 regressed spontaneously upon discontinuation of cyclosporine, while the fourth regressed despite continuation of the drug. The remainder of the malignancies, 13 cases (0.9%), involved various organs.
Patients should not be treated concurrently with cyclosporine and PUVA or UVB, other radiation therapy, or other immunosuppressive agents, because of the possibility of excessive immunosuppression and the subsequent risk of malignancies. (See CONTRAINDICATIONS) Patients should also be warned to protect themselves appropriately when in the sun, and to avoid excessive sun exposure. Patients should be thoroughly evaluated before and during treatment for the presence of malignancies, remembering that malignant lesions may be hidden by psoriatic plaques. Skin lesions not typical of psoriasis should be biopsied before starting treatment. Patients should be treated with cyclosporine [MODIFIED] only after complete resolution of suspicious lesions, and only if there are no other treatment options. (See Special Monitoring of Psoriasis Patients)
Specific Excipients
Alcohol (ethanol)
The alcohol content (See DESCRIPTION) of cyclosporine should be taken into account when given to patients in whom alcohol intake should be avoided or minimized, e.g. pregnant or breast feeding women, in patients presenting with liver disease or epilepsy, in alcoholic patients, or pediatric patients. For an adult weighing 70 kg, the maximum daily oral dose would deliver about 1 gram of alcohol (See DESCRIPTION for alcohol content of each formulation).
-
PRECAUTIONS
General
Hypertension
Cyclosporine is the active ingredient of cyclosporine capsules [MODIFIED] and cyclosporine oral solution [MODIFIED]. Hypertension is a common side effect of cyclosporine therapy which may persist. (See ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION for monitoring recommendations.) Mild or moderate hypertension is encountered more frequently than severe hypertension and the incidence decreases over time. In recipients of kidney, liver, and heart allografts treated with cyclosporine, antihypertensive therapy may be required. (See Special Monitoring of Rheumatoid Arthritis and Psoriasis Patients.) However, since cyclosporine may cause hyperkalemia, potassium-sparing diuretics should not be used. While calcium antagonists can be effective agents in treating cyclosporine-associated hypertension, they can interfere with cyclosporine metabolism. (See Drug Interactions.)
Vaccination
During treatment with cyclosporine, vaccination may be less effective; and the use of live attenuated vaccines should be avoided.
Special Monitoring of Rheumatoid Arthritis Patients
Before initiating treatment, a careful physical examination, including blood pressure measurements (on at least two occasions) and two creatinine levels to estimate baseline should be performed. Blood pressure and serum creatinine should be evaluated every 2 weeks during the initial 3 months and then monthly if the patient is stable. It is advisable to monitor serum creatinine and blood pressure always after an increase of the dose of nonsteroidal anti-inflammatory drugs and after initiation of new nonsteroidal anti-inflammatory drug therapy during cyclosporine [MODIFIED] treatment. If coadministered with methotrexate, CBC and liver function tests are recommended to be monitored monthly. (See also PRECAUTIONS, General, Hypertension)
In patients who are receiving cyclosporine, the dose of cyclosporine [MODIFIED] should be decreased by 25% to 50% if hypertension occurs. If hypertension persists, the dose of cyclosporine [MODIFIED] should be further reduced or blood pressure should be controlled with anti-hypertensive agents. In most cases, blood pressure has returned to baseline when cyclosporine was discontinued.
In placebo-controlled trials of rheumatoid arthritis patients, systolic hypertension (defined as an occurrence of two systolic blood pressure readings >140 mm Hg) and diastolic hypertension (defined as two diastolic blood pressure readings >90 mm Hg) occurred in 33% and 19% of patients treated with cyclosporine, respectively. The corresponding placebo rates were 22% and 8%.
Special Monitoring of Psoriasis Patients
Before initiating treatment, a careful dermatological and physical examination, including blood pressure measurements (on at least two occasions) should be performed. Since cyclosporine [MODIFIED] is an immunosuppressive agent, patients should be evaluated for the presence of occult infection on their first physical examination and for the presence of tumors initially, and throughout treatment with cyclosporine [MODIFIED]. Skin lesions not typical for psoriasis should be biopsied before starting cyclosporine [MODIFIED]. Patients with malignant or premalignant changes of the skin should be treated with cyclosporine [MODIFIED] only after appropriate treatment of such lesions and if no other treatment option exists.
Baseline laboratories should include serum creatinine (on two occasions), BUN, CBC, serum magnesium, potassium, uric acid, and lipids.
The risk of cyclosporine nephropathy is reduced when the starting dose is low (2.5 mg/kg/day), the maximum dose does not exceed 4 mg/kg/day, serum creatinine is monitored regularly while cyclosporine is administered, and the dose of cyclosporine [MODIFIED] is decreased when the rise in creatinine is greater than or equal to 25% above the patient's pretreatment level. The increase in creatinine is generally reversible upon timely decrease of the dose of cyclosporine [MODIFIED] or its discontinuation.
Serum creatinine and BUN should be evaluated every 2 weeks during the initial 3 months of therapy and then monthly if the patient is stable. If the serum creatinine is greater than or equal to 25% above the patient's pretreatment level, serum creatinine should be repeated within two weeks. If the change in serum creatinine remains greater than or equal to 25% above baseline, cyclosporine [MODIFIED] should be reduced by 25% to 50%. If at any time the serum creatinine increases by greater than or equal to 50% above pretreatment level, cyclosporine [MODIFIED] should be reduced by 25% to 50%. Cyclosporine [MODIFIED] should be discontinued if reversibility (within 25% of baseline) of serum creatinine is not achievable after two dosage modifications. It is advisable to monitor serum creatinine after an increase of the dose of nonsteroidal anti-inflammatory drug and after initiation of new nonsteroidal anti-inflammatory therapy during cyclosporine [MODIFIED] treatment.
Blood pressure should be evaluated every 2 weeks during the initial 3 months of therapy and then monthly if the patient is stable, or more frequently when dosage adjustments are made. Patients without a history of previous hypertension before initiation of treatment with cyclosporine [MODIFIED] should have the drug reduced by 25% to 50% if found to have sustained hypertension. If the patient continues to be hypertensive despite multiple reductions of cyclosporine [MODIFIED], then cyclosporine [MODIFIED] should be discontinued. For patients with treated hypertension, before the initiation of cyclosporine [MODIFIED] therapy, their medication should be adjusted to control hypertension while on cyclosporine [MODIFIED]. Cyclosporine [MODIFIED] should be discontinued if a change in hypertension management is not effective or tolerable.
CBC, uric acid, potassium, lipids, and magnesium should also be monitored every 2 weeks for the first 3 months of therapy, and then monthly if the patient is stable or more frequently when dosage adjustments are made. Cyclosporine [MODIFIED] dosage should be reduced by 25% to 50% for any abnormality of clinical concern.
In controlled trials of cyclosporine in psoriasis patients, cyclosporine blood concentrations did not correlate well with either improvement or with side effects such as renal dysfunction.
Information for Patients
Patients should be advised that any change of cyclosporine formulation should be made cautiously and only under physician supervision because it may result in the need for a change in dosage.
Patients should be informed of the necessity of repeated laboratory tests while they are receiving cyclosporine. Patients should be advised of the potential risks during pregnancy and informed of the increased risk of neoplasia. Patients should also be informed of the risk of hypertension and renal dysfunction.
Patients should be advised that during treatment with cyclosporine, vaccination may be less effective and the use of live attenuated vaccines should be avoided.
Patients should be given careful dosage instructions. Cyclosporine oral solution [MODIFIED] should be diluted, preferably with orange or apple juice that is at room temperature. The combination of cyclosporine oral solution [MODIFIED] with milk can be unpalatable.
Patients should be advised to take cyclosporine [MODIFIED] on a consistent schedule with regard to time of day and relation to meals. Grapefruit and grapefruit juice affect metabolism, increasing blood concentration of cyclosporine, thus should be avoided.
Cyclosporine may impact the ability to drive and use machines. Patients should be advised to exercise care when driving or using machines if they experience neurological disturbances including confusion, somnolence, or dizziness and discuss with their healthcare provider (seeWARNINGS and ADVERSE REACTIONS).
Laboratory Tests
In all patients treated with cyclosporine, renal and liver functions should be assessed repeatedly by measurement of serum creatinine, BUN, serum bilirubin, and liver enzymes. Serum lipids, magnesium, and potassium should also be monitored. Cyclosporine blood concentrations should be routinely monitored in transplant patients (see DOSAGE AND ADMINISTRATION, Blood Concentration Monitoring in Transplant Patients), and periodically monitored in rheumatoid arthritis patients.
Drug Interactions
A. Effect of Drugs and Other Agents on Cyclosporine Pharmacokinetics and/or Safety
All of the individual drugs cited below are well substantiated to interact with cyclosporine. In addition, concomitant use of nonsteroidal anti-inflammatory drugs with cyclosporine, particularly in the setting of dehydration, may potentiate renal dysfunction. Caution should be exercised when using other drugs which are known to impair renal function. (See WARNINGS, Nephrotoxicity)
Drugs That May Potentiate Renal Dysfunction
Antibiotics Antineoplastics Antifungals Anti-inflammatory Drugs ciprofloxacin
gentamicin
tobramycin
vancomycin
trimethoprim with sulfamethoxazolemelphalan amphotericin B
ketoconazoleazapropazon
colchicine
diclofenac
naproxen
sulindacGastrointestinal Agents Immunosuppressives Other Drugs cimetidine
ranitidinetacrolimus fibric acid derivatives
(e.g. bezafibrate, fenofibrate)
methotrexateDuring the concomitant use of a drug that may exhibit additive or synergistic renal impairment with cyclosporine, close monitoring of renal function (in particular serum creatinine) should be performed. If a significant impairment of renal function occurs, the dosage of the coadministered drug should be reduced or an alternative treatment considered.
Cyclosporine is extensively metabolized by CYP 3A isoenzymes, in particular CYP3A4, and is a substrate of the multidrug efflux transporter P-glycoprotein. Various agents are known to either increase or decrease plasma or whole blood concentrations of cyclosporine usually by inhibition or induction of CYP3A4 or P-glycoprotein transporter or both. Compounds that decrease cyclosporine absorption such as orlistat should be avoided. Appropriate cyclosporine [MODIFIED]dosage adjustment to achieve the desired cyclosporine concentrations is essential when drugs that significantly alter cyclosporine concentrations are used concomitantly. (See Blood Concentration Monitoring)
1. Drugs That Increase Cyclosporine Concentrations
Calcium Channel Blockers Antibiotics Other Drugs diltiazem azithromycin allopurinol nicardipine clarithromycin amiodarone verapamil erythromycin bromocriptine quinupristin/dalfopristin colchicine Antifungals danazol fluconazole Glucocorticoids imatinib itraconazole methylprednisolone metoclopramide ketoconazole nefazodone voriconazole oral contraceptives HIV protease inhibitors
The HIV protease inhibitors (e.g., indinavir, nelfinavir, ritonavir, and saquinavir) are known to inhibit cytochrome P-450 3A and thus could potentially increase the concentrations of cyclosporine, however no formal studies of the interaction are available. Care should be exercised when these drugs are administered concomitantly.
2. Drugs/Dietary Supplements That Decrease Cyclosporine Concentrations
Antibiotics Anticonvulsants Other Drugs/Dietary Supplements nafcillin
rifampincarbamazepine
oxcarbazepine
phenobarbital
phenytoinbosentan
octreotide
orlistat
sulfinpyrazone
St. John's Wort
terbinafine
ticlopidineBosentan
Coadministration of bosentan (250 - 1000 mg every 12 hours based on tolerability) and cyclosporine (300 mg every 12 hours for 2 days then dosing to achieve a Cmin of 200-250 ng/mL) for 7 days in healthy subjects resulted in decreases in the cyclosporine mean dose-normalized AUC, Cmax, and trough concentration of approximately 50%, 30% and 60%, respectively, compared to when cyclosporine was given alone (See Effect of Cyclosporine on the Pharmacokinetics and/or Safety of Other Drugs or Agents). Coadministration of cyclosporine with bosentan should be avoided.
Boceprevir
Coadministration of boceprevir (800 mg three times daily for 7 days) and cyclosporine (100 mg single dose) in healthy subjects resulted in increases in the mean AUC and Cmax of cyclosporine approximately 2.7-fold and 2-fold, respectively, compared to when cyclosporine was given alone.
Telaprevir
Coadministration of telaprevir (750 mg every 8 hours for 11 days) with cyclosporine (10 mg on day 8) in healthy subjects resulted in increases in the mean dose-normalized AUC and Cmax of cyclosporine approximately 4.5-fold and 1.3-fold, respectively, compared to when cyclosporine (100 mg single dose) was given alone.
St. John's Wort
There have been reports of a serious drug interaction between cyclosporine and the herbal dietary supplement, St. John's Wort. This interaction has been reported to produce a marked reduction in the blood concentrations of cyclosporine, resulting in subtherapeutic levels, rejection of transplanted organs, and graft loss.
B. Effect of Cyclosporine on the Pharmacokinetics and/or Safety of Other Drugs or Agents
Cyclosporine is an inhibitor of CYP3A4 and of the multidrug efflux transporter (e.g. P-glycoprotein) and may increase plasma concentrations of comedications that are substrates of CYP3A4 or P-glycoprotein or organic anion transporter proteins.
Cyclosporine may reduce the clearance of digoxin, colchicine, prednisolone, HMG-CoA reductase inhibitors (statins), and, aliskiren, bosentan, dabigatran, repaglinide, NSAIDs, sirolimus, etoposide, and other drugs.
See the full prescribing information of the other drug for further information and specific recommendations. The decision on coadministration of cyclosporine with other drugs or agents should be made by the healthcare provider following the careful assessment of benefits and risks.
Digoxin
Severe digitalis toxicity has been seen within days of starting cyclosporine in several patients taking digoxin. If digoxin is used concurrently with cyclosporine, serum digoxin concentrations should be monitored.
Colchicine
There are reports on the potential of cyclosporine to enhance the toxic effects of colchicine such as myopathy and neuropathy, especially in patients with renal dysfunction. Concomitant administration of cyclosporine and colchicine results in significant increases in colchicine plasma concentrations. If colchicine is used concurrently with cyclosporine, a reduction in the dosage of colchicine is recommended.
HMG-CoA reductase inhibitors (statins)
Literature and postmarketing cases of myotoxicity, including pain and weakness, myositis, and rhabdomyolysis, have been reported with concomitant administration of cyclosporine with lovastatin, simvastatin, atorvastatin, pravastatin, and rarely fluvastatin. When concurrently administered with cyclosporine, the dosage of these statins should be reduced according to label recommendations. Statin therapy needs to be temporarily withheld or discontinued in patients with signs and symptoms of myopathy or those with risk factors predisposing to severe renal injury, including renal failure, secondary to rhabdomyolysis.
Repaglinide
Cyclosporine may increase the plasma concentrations of repaglinide and thereby increase the risk of hypoglycemia. In 12 healthy male subjects who received two doses of 100mg cyclosporine capsule orally 12 hours apart with a single dose of 0.25 mg repaglinide tablet (one half of a 0.5 mg tablet) orally 13 hours after the cyclosporine initial dose, the repaglinide mean Cmax and AUC were increased 1.8 fold (range: 0.6 - 3.7 fold) and 2.4 fold (range 1.2 - 5.3 fold), respectively. Close monitoring of blood glucose level is advisable for a patient taking cyclosporine and repaglinide concomitantly.
Ambrisentan
Coadministration of ambrisentan (5 mg daily) and cyclosporine (100-150 mg twice daily initially, then dosing to achieve Cmin 150-200 ng/mL) for 8 days in healthy subjects resulted in mean increases in ambrisentan AUC and Cmax of approximately 2-fold and 1.5-fold, respectively, compared to ambrisentan alone. When coadministering ambrisentan with cyclosporine, the ambrisentan dose should not be titrated to the recommended maximum daily dose.
Anthracycline antibiotics
High doses of cyclosporine (e.g., at starting intravenous dose of 16 mg/kg/day) may increase the exposure to anthracycline antibiotics (e.g., doxorubicin, mitoxantrone, daunorubicin) in cancer patients.
Aliskiren
Cyclosporine alters the pharmacokinetics of aliskiren, a substrate of P-glycoprotein and CYP3A4. In 14 healthy subjects who received concomitantly single doses of cyclosporine (200 mg) and reduced dose aliskiren (75 mg), the mean Cmax of aliskiren was increased by approximately 2.5 fold (90% CI: 1.96 - 3.17) and the mean AUC by approximately 4.3 fold (90% CI: 3.52 - 5.21), compared to when these subjects received aliskiren alone. The concomitant administration of aliskiren with cyclosporine prolonged the median aliskiren elimination half-life (26 hours versus 43 to 45 hours) and the Tmax (0.5 hours versus 1.5 to 2 hours). The mean AUC and Cmax of cyclosporine were comparable to reported literature values. Coadministration of cyclosporine and aliskiren in these subjects also resulted in an increase in the number and/or intensity of adverse events, mainly headache, hot flush, nausea, vomiting, and somnolence. The coadministration of cyclosporine with aliskiren is not recommended.
Bosentan
In healthy subjects, coadministration of bosentan and cyclosporine resulted in mean increases in dose-normalized bosentan trough concentrations on day 1 and day 8 of approximately 21-fold and 2-fold , respectively, compared to when bosentan was given alone as a single dose on day 1 (See Effect of Drugs and Other Agents on Cyclosporine Pharmacokinetics and/or Safety). Coadministration of cyclosporine with bosentan should be avoided.
Dabigatran
The effect of cyclosporine on dabigatran concentrations had not been formally studied. Concomitant administration of dabigatran and cyclosporine may result in increased plasma dabigatran concentrations due to the P-gp inhibitory activity of cyclosporine. Coadministration of cyclosporine with dabigatran should be avoided.
Potassium-Sparing Diuretics
Cyclosporine should not be used with potassium-sparing diuretics because hyperkalemia can occur. Caution is also required when cyclosporine is coadministered with potassium sparing drugs (e.g. angiotensin converting enzyme inhibitors, angiotensin II receptor antagonists), potassium containing drugs as well as in patients on a potassium rich diet. Control of potassium levels in these situations is advisable.
Nonsteroidal Anti-inflammatory Drug (NSAID) Interactions
Clinical status and serum creatinine should be closely monitored when cyclosporine is used with nonsteroidal anti-inflammatory agents in rheumatoid arthritis patients. (See WARNINGS)
Pharmacodynamic interactions have been reported to occur between cyclosporine and both naproxen and sulindac, in that concomitant use is associated with additive decreases in renal function, as determined by 99mTc-diethylenetriaminepentaacetic acid (DTPA) and (p-aminohippuric acid) PAH clearances. Although concomitant administration of diclofenac does not affect blood concentrations of cyclosporine, it has been associated with approximate doubling of diclofenac blood concentrations and occasional reports of reversible decreases in renal function. Consequently, the dose of diclofenac should be in the lower end of the therapeutic range.
Methotrexate Interaction
Preliminary data indicate that when methotrexate and cyclosporine were coadministered to rheumatoid arthritis patients (N=20), methotrexate concentrations (AUCs) were increased approximately 30% and the concentrations (AUCs) of its metabolite, 7-hydroxy methotrexate, were decreased by approximately 80%. The clinical significance of this interaction is not known. Cyclosporine concentrations do not appear to have been altered (N=6).
Sirolimus
Elevations in serum creatinine were observed in studies using sirolimus in combination with full-dose cyclosporine. This effect is often reversible with cyclosporine dose reduction. Simultaneous coadministration of cyclosporine significantly increases blood levels of sirolimus. To minimize increases in sirolimus concentrations, it is recommended that sirolimus be given 4 hours after cyclosporine administration.
Nifedipine
Frequent gingival hyperplasia when nifedipine is given concurrently with cyclosporine have been reported. The concomitant use of nifedipine should be avoided in patients in whom gingival hyperplasia develops as a side effect of cyclosporine.
Methylprednisolone
Convulsions when high dose methylprednisolone is given concurrently with cyclosporine have been reported.
Other Immunosuppressive Drugs and Agents
Psoriasis patients receiving other immunosuppressive agents or radiation therapy (including PUVA and UVB) should not receive concurrent cyclosporine because of the possibility of excessive immunosuppression.
Interactions resulting in decrease of other drug levels
Cyclosporine inhibits the enterohepatic circulation of mycophenolic acid (MPA). Concomitant administration of cyclosporine and mycophenolate mofetil or mycophenolate sodium in transplant patients may decrease the mean exposure of MPA by 20 - 50% when compared with other immunosuppressants, which could reduce efficacy of mycophenolate mofetil or mycophenolate sodium. Monitor patients for alterations in efficacy of mycophenolate mofetil or mycophenolate sodium, when they are co-administered with cyclosporine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were carried out in male and female rats and mice. In the 78-week mouse study, evidence of a statistically significant trend was found for lymphocytic lymphomas in females, and the incidence of hepatocellular carcinomas in mid-dose(0.03 times the maximum recommended human dose (MRHD) based on body surface area (BSA) males significantly exceeded the control value. In the 24-month rat study, pancreatic islet cell adenomas significantly exceeded the control rate in the low dose level (0.006 times the MRHD based on BSA). The hepatocellular carcinomas and pancreatic islet cell adenomas were not dose related. Published reports indicate that co-treatment of hairless mice with UV irradiation and cyclosporine or other immunosuppressive agents shorten the time to skin tumor formation compared to UV irradiation alone.
Cyclosporine has not been found to be mutagenic/genotoxic in the Ames Test, the V79-HGPRT Test, the micronucleus test in mice and Chinese hamsters, the chromosome-aberration tests in Chinese hamster bone-marrow, the mouse dominant lethal assay, and the DNA-repair test in sperm from treated mice. A recent study analyzing sister chromatid exchange (SCE) induction by cyclosporine using human lymphocytes in vitro gave indication of a positive effect (i.e., induction of SCE), at high concentrations in this system.
In a fertility study in rats, increased perinatal mortality and impaired postnatal development of F1 pups were observed at 15 mg/kg/day (0.2 times the MRHD based on BSA). No adverse effects on fertility and reproduction were observed up to 5 mg/kg/day (0.06 times the MRHD based on BSA) in male and female rats.
Widely distributed papillomatosis of the skin was observed after chronic treatment of dogs with cyclosporine at 9 times the human initial psoriasis treatment dose of 2.5 mg/kg, where doses are expressed on a body surface area basis. This papillomatosis showed a spontaneous regression upon discontinuation of cyclosporine.
An increased incidence of malignancy is a recognized complication of immunosuppression in recipients of organ transplants and patients with rheumatoid arthritis and psoriasis. The most common forms of neoplasms are non-Hodgkin's lymphoma and carcinomas of the skin. The risk of malignancies in cyclosporine recipients is higher than in the normal, healthy population but similar to that in patients receiving other immunosuppressive therapies. Reduction or discontinuance of immunosuppression may cause the lesions to regress.
In psoriasis patients on cyclosporine, development of malignancies, especially those of the skin has been reported. (See WARNINGS) Skin lesions not typical for psoriasis should be biopsied before starting cyclosporine treatment. Patients with malignant or premalignant changes of the skin should be treated with cyclosporine only after appropriate treatment of such lesions and if no other treatment option exists.
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to cyclosporine during pregnancy. Encourage women who are taking cyclosporine during pregnancy to enroll in the Transplant Pregnancy Registry International (TPRI) by calling 1-877-955-8677 or visiting https://www.transplantpregnancyregistry.org.
Risk Summary
Available data from published literature, including the Transplant Pregnancy Registry International, observational cohort studies, case-controlled studies, meta-analysis, case series, and case reports, over decades of use with cyclosporine in pregnancy have not identified a drug associated risk of major birth defects, or miscarriage. Adverse maternal or fetal outcomes including hypertension, preeclampsia, preterm birth, and low birth weight are increased in patients treated with cyclosporine. However, patients receiving cyclosporine during pregnancy have underlying medical conditions and may be treated with concomitant medications that limit the interpretability of these findings (seeData). Embryo-fetal developmental (EFD) studies in rats and rabbits with cyclosporine have shown embryo-fetal toxicity at dose levels below the maximum recommended human dose (MRHD) based on body surface area (BSA). The alcohol content of cyclosporine should be taken into account when given to pregnant women (see WARNINGS, Special Excipients). The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Available data from the National Transplantation Pregnancy Registry (NTPR) including 622 pregnancies in renal, liver, and heart transplant recipients exposed to cyclosporine during pregnancy found that the overall rate of major birth defects, live birth rates, and miscarriage rates were comparable to the general population. Maternal and fetal adverse outcomes, including the rate of hypertension, preeclampsia, premature births, and low birth weight infants appear to be increased in transplant recipients treated with cyclosporine compared to the general population. However, these patients have underlying medical conditions that confound the above findings.
Animal Data
Animal studies have shown reproductive toxicity in rats and rabbits.
Three EFD studies (two oral and one intravenous) are available in rats. In two EFD studies, pregnant rats were orally administered with cyclosporine either at doses of 10, 17, 30, 100 and 300 mg/kg/day or 4, 10 and 25 mg/kg/day from gestation day (GD) 6 to 15 or from GD 7 to 17, respectively. Maternal toxicity characterized by mortality, clinical signs of toxicity and impaired body weight gain were observed at 30 mg/kg/day and above. Cyclosporine was embryo- and fetotoxic as indicated by increased embryonic mortality and reduced fetal weight together with skeletal retardations in rats at 25 mg/kg/day and above. In addition, ventricular septal defect was observed at 25 mg/kg/day in fetuses. In the first study, the oral no observed effect level (NOEL) for both dams and fetuses was 17 mg/kg/day (0.2 times the MRHD based on BSA). In the other oral study, the NOEL for dams and fetuses were 10 and 4 mg/kg/day (0.13 and 0.05 times the MRHD based on BSA), respectively. In the IV EFD study, rats were administered with 3, 6 and 12 mg/kg/day of cyclosporine from GD 7 to 17. An increase in post implantation loss was observed at 12 mg/kg/day; ventricular septal defect was observed at ≥ 6 mg/kg/day in fetuses. The IV NOEL for dams and fetus were 6 and 3 mg/kg/day (0.08 and 0.04 times the MRHD, respectively, based on BSA), respectively, after IV administration.
In rabbits, cyclosporine was orally administered at dose levels of 10, 30, 100 or 300 mg/kg/day from GD 6 to 18. At 100 mg/kg/day and above, reduction in body weight gain of dams and at 300 mg/kg/day abortions were observed. Maternal toxicity, embryo-fetotoxicity as indicated by increased pre- and postnatal mortality, reduced fetal weight together with skeletal retardations were observed at 100 mg/kg/day and above. The NOEL for dams and fetuses was 30 mg/kg/day (1 times the MRHD based on BSA).
In two published research studies, rabbits exposed to cyclosporine in utero (10 mg/kg/day subcutaneously) demonstrated reduced numbers of nephrons, renal hypertrophy, systemic hypertension and progressive renal insufficiency up to 35 weeks of age. These findings have not been demonstrated in other species and their relevance for humans is unknown.
In a peri- and postnatal development study in rats, pregnant rats were orally administered with cyclosporine (5, 15 or 45 mg/kg/day) from GD 15 until end of lactation. At 45 mg/kg/day (0.5 times the MRHD based on BSA), increased pre and postnatal mortality of offspring and reduced body weight gain of surviving pups were observed. Cyclosporine up to 15 mg/kg/day (0.2 times the MRHD based on BSA) had no effect on pregnancy, pre and postnatal development of offspring.
Nursing Mothers
Risk Summary
Cyclosporine and its metabolites are present in human milk following oral and intravenous administration. Adverse effects on the breastfed infant have not been reported. There are no data on the effects of the drug on milk production. The alcohol content of NEORAL should be taken into account when given to lactating women (see WARNINGS, Special Excipients). Lactating women are encouraged to avoid additional alcohol intake during treatment. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for NEORAL and any potential adverse effects on the breastfed infant from NEORAL or from the underlying maternal condition.
Pediatric Use
Although no adequate and well-controlled studies have been completed in children, transplant recipients as young as one year of age have received cyclosporine [MODIFIED] with no unusual adverse effects. The safety and efficacy of cyclosporine [MODIFIED] treatment in children with juvenile rheumatoid arthritis or psoriasis below the age of 18 have not been established.
Geriatric Use
In rheumatoid arthritis clinical trials with cyclosporine, 17.5% of patients were age 65 or older. These patients were more likely to develop systolic hypertension on therapy, and more likely to show serum creatinine rises greater than or equal to 50% above the baseline after 3 to 4 months of therapy.
Clinical studies of cyclosporine [MODIFIED] in transplant and psoriasis patients did not include a sufficient number of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experiences have not identified differences in response between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Kidney, Liver, and Heart Transplantation
The principal adverse reactions of cyclosporine therapy are renal dysfunction, tremor, hirsutism, hypertension, and gum hyperplasia.
Hypertension
Hypertension, which is usually mild to moderate, may occur in approximately 50% of patients following renal transplantation and in most cardiac transplant patients.
Glomerular Capillary Thrombosis
Glomerular capillary thrombosis has been found in patients treated with cyclosporine and may progress to graft failure. The pathologic changes resembled those seen in the hemolytic-uremic syndrome and included thrombosis of the renal microvasculature, with platelet-fibrin thrombi occluding glomerular capillaries and afferent arterioles, microangiopathic hemolytic anemia, thrombocytopenia, and decreased renal function. Similar findings have been observed when other immunosuppressives have been employed post-transplantation.
Hypomagnesemia
Hypomagnesemia has been reported in some, but not all, patients exhibiting convulsions while on cyclosporine therapy. Although magnesium depletion studies in normal subjects suggest that hypomagnesemia is associated with neurologic disorders, multiple factors, including hypertension, high dose methylprednisolone, hypocholesterolemia, and nephrotoxicity associated with high plasma concentrations of cyclosporine appear to be related to the neurological manifestations of cyclosporine toxicity.
Clinical Studies
In controlled studies, the nature, severity, and incidence of the adverse events that were observed in 493 transplanted patients treated with cyclosporine [MODIFIED] were comparable with those observed in 208 transplanted patients who received Sandimmune® (cyclosporine) in these same studies when the dosage of the two drugs was adjusted to achieve the same cyclosporine blood trough concentrations.
Based on the historical experience with Sandimmune®, the following reactions occurred in 3% or greater of 892 patients involved in clinical trials of kidney, heart, and liver transplants.
Randomized Kidney Patients Cyclosporine Patients
(Sandimmune®)Body System Adverse Reactions Sandimmune®
(N=227)%Azathioprine
(N=228)%Kidney
(N=705)%Heart
(N=112)%Liver
(N=75)%Genitourinary Renal Dysfunction 32 6 25 38 37 Cardiovascular Hypertension 26 18 13 53 27 Cramps 4 <1 2 <1 0 Skin Hirsutism 21 <1 21 28 45 Acne 6 8 2 2 1 Central Nervous System Tremor 12 0 21 31 55 Convulsions 3 1 1 4 5 Headache 2 <1 2 15 4 Gastrointestinal Gum Hyperplasia 4 0 9 5 16 Diarrhea 3 <1 3 4 8 Nausea/Vomiting 2 <1 4 10 4 Hepatotoxicity <1 <1 4 7 4 Abdominal Discomfort <1 0 <1 7 0 Autonomic Nervous System Paresthesia 3 0 1 2 1 Flushing <1 0 4 0 4 Hematopoietic Leukopenia 2 19 <1 6 0 Lymphoma <1 0 1 6 1 Respiratory Sinusitis <1 0 4 3 7 Miscellaneous Gynecomastia <1 0 <1 4 3 Among 705 kidney transplant patients treated with cyclosporine oral solution (Sandimmune®) in clinical trials, the reason for treatment discontinuation was renal toxicity in 5.4%, infection in 0.9%, lack of efficacy in 1.4%, acute tubular necrosis in 1%, lymphoproliferative disorders in 0.3%, hypertension in 0.3%, and other reasons in 0.7% of the patients.
The following reactions occurred in 2% or less of cyclosporine-treated patients: allergic reactions, anemia, anorexia, confusion, conjunctivitis, edema, fever, brittle fingernails, gastritis, hearing loss, hiccups, hyperglycemia, migraine (cyclosporine [MODIFIED]) muscle pain, peptic ulcer, thrombocytopenia, tinnitus.
The following reactions occurred rarely: anxiety, chest pain, constipation, depression, hair breaking, hematuria, joint pain, lethargy, mouth sores, myocardial infarction, night sweats, pancreatitis, pruritus, swallowing difficulty, tingling, upper GI bleeding, visual disturbance, weakness, weight loss.
Patients receiving immunosuppressive therapies, including cyclosporine and cyclosporine -containing regimens, are at increased risk of infections (viral, bacterial, fungal, parasitic). Both generalized and localized infections can occur. Pre-existing infections may also be aggravated. Fatal outcomes have been reported (see Warnings).
Infectious Complication In Historical Randomized Studies In Renal Transplant Patients Using Sandimmune® Complication Cyclosporine Treatment
(N=227)Azathioprine with Steroids*
(N=228)% of Complications % of Complications - *
- Some patients also received ALG.
Septicemia 5.3 4.8 Abscesses 4.4 5.3 Systemic Fungal Infection 2.2 3.9 Local Fungal Infection 7.5 9.6 Cytomegalovirus 4.8 12.3 Other Viral Infections 15.9 18.4 Urinary Tract Infections 21.1 20.2 Wound and Skin Infections 7 10.1 Pneumonia 6.2 9.2 Postmarketing Experience, Kidney, Liver and Heart Transplantation
Hepatotoxicity
Cases of hepatotoxicity and liver injury including cholestasis, jaundice, hepatitis and liver failure; serious and/or fatal outcomes have been reported (See WARNINGS/Hepatotoxicity).
Increased Risk of Infections
Cases of JC virus-associated progressive multifocal leukoencephalopathy (PML), sometimes fatal; and polyoma virus-associated nephropathy (PVAN), especially BK virus resulting in graft loss have been reported. (See WARNINGS/ Polyoma Virus Infection)
Rheumatoid Arthritis
The principal adverse reactions associated with the use of cyclosporine in rheumatoid arthritis are renal dysfunction (see WARNINGS), hypertension (see PRECAUTIONS), headache, gastrointestinal disturbances, and hirsutism/hypertrichosis.
In rheumatoid arthritis patients treated in clinical trials within the recommended dose range, cyclosporine therapy was discontinued in 5.3% of the patients because of hypertension and in 7% of the patients because of increased creatinine. These changes are usually reversible with timely dose decrease or drug discontinuation. The frequency and severity of serum creatinine elevations increase with dose and duration of cyclosporine therapy. These elevations are likely to become more pronounced without dose reduction or discontinuation.
The following adverse events occurred in controlled clinical trials:
Cyclosporine [MODIFIED]/Sandimmune® (cyclosporine) Rheumatoid Arthritis Percentage of Patients with Adverse Events ≥ 3% in any Cyclosporine Treated Group Studies
651+652+2008Study
302Study
654Study
654Study
302Studies
651+652+2008Body System Preferred Term Sandimmune® *
(N=269)Sandimmune®
(N=155)Methotrexate & Sandimmune®
(N=74)Methotrexate & Placebo
(N=73)Cyclosporine [MODIFIED]
(N=143)Placebo
(N=201)Autonomic Nervous System Disorders Flushing 2% 2% 3% 0% 5% 2% Body As A Whole — General Disorders Accidental Trauma 0% 1% 10% 4% 4% 0% Edema NOS† 5% 14% 12% 4% 10% <1% Fatigue 6% 3% 8% 12% 3% 7% Fever 2% 3% 0% 0% 2% 4% Influenza-like symptoms <1% 6% 1% 0% 3% 2% Pain 6% 9% 10% 15% 13% 4% Rigors 1% 1% 4% 0% 3% 1% Cardiovascular Disorders Arrhythmia 2% 5% 5% 6% 2% 1% Chest Pain 4% 5% 1% 1% 6% 1% Hypertension 8% 26% 16% 12% 25% 2% Central and Peripheral Nervous System Disorders Dizziness 8% 6% 7% 3% 8% 3% Headache 17% 23% 22% 11% 25% 9% Migraine 2% 3% 0% 0% 3% 1% Paresthesia 8% 7% 8% 4% 11% 1% Tremor 8% 7% 7% 3% 13% 4% Gastrointestinal System Disorders Abdominal Pain 15% 15% 15% 7% 15% 10% Anorexia 3% 3% 1% 0% 3% 3% Diarrhea 12% 12% 18% 15% 13% 8% Dyspepsia 12% 12% 10% 8% 8% 4% Flatulence 5% 5% 5% 4% 4% 1% Gastrointestinal Disorder NOS 0% 2% 1% 4% 4% 0% Gingivitis 4% 3% 0% 0% 0% 1% Gum Hyperplasia 2% 4% 1% 3% 4% 1% Nausea 23% 14% 24% 15% 18% 14% Rectal Hemorrhage 0% 3% 0% 0% 1% 1% Stomatitis 7% 5% 16% 12% 6% 8% Vomiting 9% 8% 14% 7% 6% 5% Hearing and Vestibular Disorders Ear Disorder NOS 0% 5% 0% 0% 1% 0% Metabolic and Nutritional Disorders Hypomagnesemia 0% 4% 0% 0% 6% 0% Musculoskeletal System Disorders Arthropathy 0% 5% 0% 1% 4% 0% Leg Cramps/Involuntary Muscle Contractions 2% 11% 11% 3% 12% 1% Psychiatric Disorders Depression 3% 6% 3% 1% 1% 2% Insomnia 4% 1% 1% 0% 3% 2% Renal Creatinine elevations ≥30% 43% 39% 55% 19% 48% 13% Creatinine elevations ≥50% 24% 18% 26% 8% 18% 3% Reproductive Disorders, Female Leukorrhea 1% 0% 4% 0% 1% 0% Menstrual Disorder 3% 2% 1% 0% 1% 1% Respiratory System Disorders Bronchitis 1% 3% 1% 0% 1% 3% Coughing 5% 3% 5% 7% 4% 4% Dyspnea 5% 1% 3% 3% 1% 2% Infection NOS 9% 5% 0% 7% 3% 10% Pharyngitis 3% 5% 5% 6% 4% 4% Pneumonia 1% 0% 4% 0% 1% 1% Rhinitis 0% 3% 11% 10% 1% 0% Sinusitis 4% 4% 8% 4% 3% 3% Upper Respiratory Tract 0% 14% 23% 15% 13% 0% Skin and Appendages Disorders Alopecia 3% 0% 1% 1% 4% 4% Bullous Eruption 1% 0% 4% 1% 1% 1% Hypertrichosis 19% 17% 12% 0% 15% 3% Rash 7% 12% 10% 7% 8% 10% Skin Ulceration 1% 1% 3% 4% 0% 2% Urinary System Disorders Dysuria 0% 0% 11% 3% 1% 2% Micturition Frequency 2% 4% 3% 1% 2% 2% NPN, Increased 0% 19% 12% 0% 18% 0% Urinary Tract Infection 0% 3% 5% 4% 3% 0% Vascular (Extracardiac) Disorders Purpura 3% 4% 1% 1% 2% 0% In addition, the following adverse events have been reported in 1% to <3% of the rheumatoid arthritis patients in the cyclosporine treatment group in controlled clinical trials.
Autonomic Nervous System: dry mouth, increased sweating
Body as a Whole: allergy, asthenia, hot flushes, malaise, overdose, procedure NOS2, tumor NOS, weight decrease, weight increase
Cardiovascular: abnormal heart sounds, cardiac failure, myocardial infarction, peripheral ischemia
Central and Peripheral Nervous System: hypoesthesia, neuropathy, vertigo
Endocrine: goiter
Gastrointestinal: constipation, dysphagia, enanthema, eructation, esophagitis, gastric ulcer, gastritis, gastroenteritis, gingival bleeding, glossitis, peptic ulcer, salivary gland enlargement, tongue disorder, tooth disorder
Infection: abscess, bacterial infection, cellulitis, folliculitis, fungal infection, herpes simplex, herpes zoster, renal abscess, moniliasis, tonsillitis, viral infection
Hematologic: anemia, epistaxis, leukopenia, lymphadenopathy
Liver and Biliary System: bilirubinemia
Metabolic and Nutritional: diabetes mellitus, hyperkalemia, hyperuricemia, hypoglycemia
Musculoskeletal System: arthralgia, bone fracture, bursitis, joint dislocation, myalgia, stiffness, synovial cyst, tendon disorder
Neoplasms: breast fibroadenosis, carcinoma
Psychiatric: anxiety, confusion, decreased libido, emotional lability, impaired concentration, increased libido, nervousness, paroniria, somnolence
Reproductive (Female): breast pain, uterine hemorrhage
Respiratory System: abnormal chest sounds, bronchospasm
Skin and Appendages: abnormal pigmentation, angioedema, dermatitis, dry skin, eczema, nail disorder, pruritus, skin disorder, urticaria
Special Senses: abnormal vision, cataract, conjunctivitis, deafness, eye pain, taste perversion, tinnitus, vestibular disorder
Urinary System: abnormal urine, hematuria, increased BUN, micturition urgency, nocturia, polyuria, pyelonephritis, urinary incontinence
- 2
- NOS = Not Otherwise Specified
Psoriasis
The principal adverse reactions associated with the use of cyclosporine in patients with psoriasis are renal dysfunction, headache, hypertension, hypertriglyceridemia, hirsutism/hypertrichosis, paresthesia or hyperesthesia, influenza-like symptoms, nausea/vomiting, diarrhea, abdominal discomfort, lethargy, and musculoskeletal or joint pain.
In psoriasis patients treated in U.S. controlled clinical studies within the recommended dose range, cyclosporine therapy was discontinued in 1% of the patients because of hypertension and in 5.4% of the patients because of increased creatinine. In the majority of cases, these changes were reversible after dose reduction or discontinuation of cyclosporine.
There has been one reported death associated with the use of cyclosporine in psoriasis. A 27-year-old male developed renal deterioration and was continued on cyclosporine. He had progressive renal failure leading to death.
Frequency and severity of serum creatinine increases with dose and duration of cyclosporine therapy. These elevations are likely to become more pronounced and may result in irreversible renal damage without dose reduction or discontinuation.
Adverse Events Occurring in 3% or More of Psoriasis Patients in Controlled Clinical Trials Body System* Preferred Term Cyclosporine [MODIFIED] (N=182) Sandimmune®
(N=185)Infection or Potential Infection 24.7% 24.3% Influenza-like Symptoms 9.9% 8.1% Upper Respiratory Tract Infections 7.7% 11.3% Cardiovascular System 28% 25.4% Hypertension† 27.5% 25.4% Urinary System 24.2% 16.2% Increased Creatinine 19.8% 15.7% Central and Peripheral Nervous System 26.4% 20.5% Headache 15.9% 14% Paresthesia 7.1% 4.8% Musculoskeletal System 13.2% 8.7% Arthralgia 6% 1.1% Body as a Whole — General 29.1% 22.2% Pain 4.4% 3.2% Metabolic and Nutritional 9.3% 9.7% Reproductive, Female 8.5% (4 of 47 females) 11.5% (6 of 52 females) Resistance Mechanism 18.7% 21.1% Skin and Appendages 17.6% 15.1% Hypertrichosis 6.6% 5.4% Respiratory System 5% 6.5% Bronchospasm, Coughing, Dyspnea, Rhinitis 5% 4.9% Psychiatric 5% 3.8% Gastrointestinal System 19.8% 28.7% Abdominal Pain 2.7% 6% Diarrhea 5% 5.9% Dyspepsia 2.2% 3.2% Gum Hyperplasia 3.8% 6% Nausea 5.5% 5.9% White cell and RES 4.4% 2.7% The following events occurred in 1% to less than 3% of psoriasis patients treated with cyclosporine:
Body as a Whole: fever, flushes, hot flushes
Cardiovascular: chest pain
Central and Peripheral Nervous System: appetite increased, insomnia, dizziness, nervousness, vertigo
Gastrointestinal: abdominal distention, constipation, gingival bleeding
Liver and Biliary System: hyperbilirubinemia
Neoplasms: skin malignancies [squamous cell (0.9%) and basal cell (0.4%) carcinomas]
Reticuloendothelial: platelet, bleeding, and clotting disorders, red blood cell disorder
Respiratory: infection, viral and other infection
Skin and Appendages: acne, folliculitis, keratosis, pruritus, rash, dry skin
Urinary System: micturition frequency
Vision: abnormal vision
Mild hypomagnesemia and hyperkalemia may occur but are asymptomatic. Increases in uric acid may occur and attacks of gout have been rarely reported. A minor and dose related hyperbilirubinemia has been observed in the absence of hepatocellular damage. Cyclosporine therapy may be associated with a modest increase of serum triglycerides or cholesterol. Elevations of triglycerides (>750 mg/dL) occur in about 15% of psoriasis patients; elevations of cholesterol (>300 mg/dL) are observed in less than 3% of psoriasis patients. Generally these laboratory abnormalities are reversible upon dose reduction or discontinuation of cyclosporine.
-
OVERDOSAGE
There is a minimal experience with cyclosporine overdosage. Forced emesis can be of value up to 2 hours after administration of cyclosporine [MODIFIED]. Transient hepatotoxicity and nephrotoxicity may occur which should resolve following drug withdrawal. Oral doses of cyclosporine up to 10 g (about 150 mg/kg) have been tolerated with relatively minor clinical consequences, such as vomiting, drowsiness, headache, tachycardia and, in a few patients, moderately severe, reversible impairment of renal function. However, serious symptoms of intoxication have been reported following accidental parenteral overdosage with cyclosporine in premature neonates. General supportive measures and symptomatic treatment should be followed in all cases of overdosage. Cyclosporine is not dialyzable to any great extent, nor is it cleared well by charcoal hemoperfusion. The oral dosage at which half of experimental animals are estimated to die is 31 times, 39 times, and >54 times the human maintenance dose for transplant patients (6 mg/kg; corrections based on body surface area) in mice, rats, and rabbits.
-
DOSAGE AND ADMINISTRATION
Cyclosporine Capsules [Modified] (Soft Gelatin) and CYCLOSPORINE ORAL SOLUTION [MODIFIED]
Cyclosporine capsules [MODIFIED] and cyclosporine oral solution [MODIFIED] have increased bioavailability in comparison to Sandimmune® (cyclosporine capsules and cyclosporine oral solution). Cyclosporine [MODIFIED] and Sandimmune® are not bioequivalent and cannot be used interchangeably without physician supervision.
The daily dose of cyclosporine [MODIFIED] should always be given in two divided doses (b.i.d.). It is recommended that cyclosporine [MODIFIED] be administered on a consistent schedule with regard to time of day and relation to meals. Grapefruit and grapefruit juice affect metabolism, increasing blood concentration of cyclosporine, thus should be avoided.
Specific Populations
Renal Impairment in Kidney, Liver and Heart Transplantation
Cyclosporine undergoes minimal renal elimination and its pharmacokinetics do not appear to be significantly altered in patients with end-stage renal disease who receive routine hemodialysis treatments (See CLINICAL PHARMACOLOGY). However, due to its nephrotoxic potential (See WARNINGS), careful monitoring of renal function is recommended; cyclosporine dosage should be reduced if indicated. (See WARNINGS and PRECAUTIONS)
Renal Impairment in Rheumatoid Arthritis and Psoriasis
Patients with impaired renal function should not receive cyclosporine. (see CONTRAINDICATIONS, WARNINGS and PRECAUTIONS)
Hepatic Impairment
The clearance of cyclosporine may be significantly reduced in severe liver disease patients (See CLINICAL PHARMACOLOGY). Dose reduction may be necessary in patients with severe liver impairment to maintain blood concentrations within the recommended target range. (See WARNINGS and PRECAUTIONS)
Newly Transplanted Patients
The initial oral dose of cyclosporine [MODIFIED] can be given 4 to 12 hours prior to transplantation or be given postoperatively. The initial dose of cyclosporine [MODIFIED] varies depending on the transplanted organ and the other immunosuppressive agents included in the immunosuppressive protocol. In newly transplanted patients, the initial oral dose of cyclosporine [MODIFIED] is the same as the initial oral dose of Sandimmune®. Suggested initial doses are available from the results of a 1994 survey of the use of Sandimmune® in U.S. transplant centers. The mean ± SD initial doses were 9±3 mg/kg/day for renal transplant patients (75 centers), 8±4 mg/kg/day for liver transplant patients (30 centers), and 7±3 mg/kg/day for heart transplant patients (24 centers). Total daily doses were divided into two equal daily doses. The cyclosporine [MODIFIED] dose is subsequently adjusted to achieve a pre-defined cyclosporine blood concentration. (See Blood Concentration Monitoring in Transplant Patients, below) If cyclosporine trough blood concentrations are used, the target range is the same for cyclosporine [MODIFIED] as for Sandimmune®. Using the same trough concentration target range for cyclosporine [MODIFIED] as for Sandimmune® results in greater cyclosporine exposure when cyclosporine [MODIFIED] is administered. (See Pharmacokinetics, Absorption) Dosing should be titrated based on clinical assessments of rejection and tolerability. Lower cyclosporine [MODIFIED] doses may be sufficient as maintenance therapy.
Adjunct therapy with adrenal corticosteroids is recommended initially. Different tapering dosage schedules of prednisone appear to achieve similar results. A representative dosage schedule based on the patient's weight started with 2 mg/kg/day for the first 4 days tapered to 1 mg/kg/day by 1 week, 0.6 mg/kg/day by 2 weeks, 0.3 mg/kg/day by 1 month, and 0.15 mg/kg/day by 2 months and thereafter as a maintenance dose. Steroid doses may be further tapered on an individualized basis depending on status of patient and function of graft. Adjustments in dosage of prednisone must be made according to the clinical situation.
Conversion from Sandimmune® (cyclosporine to cyclosporine [MODIFIED] in Transplant Patients
In transplanted patients who are considered for conversion to cyclosporine [MODIFIED] from Sandimmune®, cyclosporine [MODIFIED] should be started with the same daily dose as was previously used with Sandimmune® (1:1 dose conversion). The cyclosporine [MODIFIED] dose should subsequently be adjusted to attain the pre-conversion cyclosporine blood trough concentration. Using the same trough concentration target range for cyclosporine [MODIFIED] as for Sandimmune® results in greater cyclosporine exposure when cyclosporine [MODIFIED] is administered. (See Pharmacokinetics, Absorption) Patients with suspected poor absorption of Sandimmune® require different dosing strategies. (See Transplant Patients with Poor Absorption of Sandimmune®). In some patients, the increase in blood trough concentration is more pronounced and may be of clinical significance.
Until the blood trough concentration attains the pre-conversion value, it is strongly recommended that the cyclosporine blood trough concentration be monitored every 4 to 7 days after conversion to cyclosporine [MODIFIED]. In addition, clinical safety parameters such as serum creatinine and blood pressure should be monitored every two weeks during the first two months after conversion. If the blood trough concentrations are outside the desired range and/or if the clinical safety parameters worsen, the dosage of cyclosporine [MODIFIED] must be adjusted accordingly.
Transplant Patients with Poor Absorption of Sandimmune®
Patients with lower than expected cyclosporine blood trough concentrations in relation to the oral dose of Sandimmune® may have poor or inconsistent absorption of cyclosporine from Sandimmune®. After conversion to cyclosporine [MODIFIED], patients tend to have higher cyclosporine concentrations. Due to the increase in bioavailability of cyclosporine following conversion to cyclosporine [MODIFIED], the cyclosporine blood trough concentration may exceed the target range. Particular caution should be exercised when converting patients to cyclosporine [MODIFIED] at doses greater than 10 mg/kg/day. The dose of cyclosporine [MODIFIED] should be titrated individually based on cyclosporine trough concentrations, tolerability, and clinical response. In this population the cyclosporine blood trough concentration should be measured more frequently, at least twice a week (daily, if initial dose exceeds 10 mg/kg/day) until the concentration stabilizes within the desired range.
Rheumatoid Arthritis
The initial dose of cyclosporine [MODIFIED] is 2.5 mg/kg/day, taken twice daily as a divided (b.i.d.) oral dose. Salicylates, nonsteroidal anti-inflammatory agents, and oral corticosteroids may be continued. (See WARNINGS and PRECAUTIONS: Drug Interactions) Onset of action generally occurs between 4 and 8 weeks. If insufficient clinical benefit is seen and tolerability is good (including serum creatinine less than 30% above baseline), the dose may be increased by 0.5 to 0.75 mg/kg/day after 8 weeks and again after 12 weeks to a maximum of 4 mg/kg/day. If no benefit is seen by 16 weeks of therapy, cyclosporine [MODIFIED] therapy should be discontinued.
Dose decreases by 25% to 50% should be made at any time to control adverse events, e.g., hypertension, elevations in serum creatinine (30% above patient's pretreatment level) or clinically significant laboratory abnormalities. (See WARNINGS and PRECAUTIONS)
If dose reduction is not effective in controlling abnormalities or if the adverse event or abnormality is severe, cyclosporine [MODIFIED] should be discontinued. The same initial dose and dosage range should be used if cyclosporine [MODIFIED] is combined with the recommended dose of methotrexate. Most patients can be treated with cyclosporine [MODIFIED] doses of 3 mg/kg/day or below when combined with methotrexate doses of up to 15 mg/week. (See CLINICAL PHARMACOLOGY, Clinical Trials)
There is limited long-term treatment data. Recurrence of rheumatoid arthritis disease activity is generally apparent within 4 weeks after stopping cyclosporine.
Psoriasis
The initial dose of cyclosporine [MODIFIED] should be 2.5 mg/kg/day. Cyclosporine [MODIFIED] should be taken twice daily, as a divided (1.25 mg/kg b.i.d.) oral dose. Patients should be kept at that dose for at least 4 weeks, barring adverse events. If significant clinical improvement has not occurred in patients by that time, the patient's dosage should be increased at 2 week intervals. Based on patient response, dose increases of approximately 0.5 mg/kg/day should be made to a maximum of 4 mg/kg/day.
Dose decreases by 25% to 50% should be made at any time to control adverse events, e.g., hypertension, elevations in serum creatinine (≥ 25% above the patient's pretreatment level), or clinically significant laboratory abnormalities. If dose reduction is not effective in controlling abnormalities, or if the adverse event or abnormality is severe, cyclosporine [MODIFIED] should be discontinued. (See Special Monitoring of Psoriasis Patients)
Patients generally show some improvement in the clinical manifestations of psoriasis in 2-weeks. Satisfactory control and stabilization of the disease may take 12 to 16 weeks to achieve. Results of a dose-titration clinical trial with cyclosporine [MODIFIED] indicate that an improvement of psoriasis by 75% or more (based on PASI) was achieved in 51% of the patients after 8 weeks and in 79% of the patients after 16 weeks. Treatment should be discontinued if satisfactory response cannot be achieved after 6 weeks at 4 mg/kg/day or the patient's maximum tolerated dose. Once a patient is adequately controlled and appears stable the dose of cyclosporine [MODIFIED] should be lowered, and the patient treated with the lowest dose that maintains an adequate response (this should not necessarily be total clearing of the patient). In clinical trials, cyclosporine doses at the lower end of the recommended dosage range were effective in maintaining a satisfactory response in 60% of the patients. Doses below 2.5 mg/kg/day may also be equally effective.
Upon stopping treatment with cyclosporine, relapse will occur in approximately 6 weeks (50% of the patients) to 16 weeks (75% of the patients). In the majority of patients rebound does not occur after cessation of treatment with cyclosporine. Thirteen cases of transformation of chronic plaque psoriasis to more severe forms of psoriasis have been reported. There were 9 cases of pustular and 4 cases of erythrodermic psoriasis. Long-term experience with cyclosporine [MODIFIED] in psoriasis patients is limited and continuous treatment for extended periods greater than one year is not recommended. Alternation with other forms of treatment should be considered in the long-term management of patients with this life-long disease.
Cyclosporine oral solution [MODIFIED]
Recommendations for Administration
To make cyclosporine oral solution [MODIFIED] more palatable, it should be diluted, with orange or apple juice that is at room temperature. Patients should avoid switching diluents frequently. Grapefruit juice affects metabolism of cyclosporine and should be avoided. The combination of cyclosporine oral solution [MODIFIED] with milk can be unpalatable. The effect of milk on the bioavailability of cyclosporine when administered as cyclosporine oral solution [MODIFIED] has not been evaluated.
Take the prescribed amount of cyclosporine oral solution [MODIFIED] from the container using the dosing syringe supplied, after removal of the protective cover, and transfer the solution to a glass of orange or apple juice. Stir well and drink at once. Do not allow diluted oral solution to stand before drinking. Use a glass container (not plastic). Rinse the glass with more diluent to ensure that the total dose is consumed. After use, dry the outside of the dosing syringe with a clean towel and replace the protective cover. Do not rinse the dosing syringe with water or other cleaning agents. If the syringe requires cleaning, it must be completely dry before resuming use.
Blood Concentration Monitoring in Transplant Patients
Transplant centers have found blood concentration monitoring of cyclosporine to be an essential component of patient management. Of importance to blood concentration analysis are the type of assay used, the transplanted organ, and other immunosuppressant agents being administered. While no fixed relationship has been established, blood concentration monitoring may assist in the clinical evaluation of rejection and toxicity, dose adjustments, and the assessment of compliance.
Various assays have been used to measure blood concentrations of cyclosporine. Older studies using a nonspecific assay often cited concentrations that were roughly twice those of the specific assays. Therefore, comparison between concentrations in the published literature and an individual patient concentration using current assays must be made with detailed knowledge of the assay methods employed. Current assay results are also not interchangeable and their use should be guided by their approved labeling. A discussion of the different assay methods is contained in Annals of Clinical Biochemistry 1994;31:420-446. While several assays and assay matrices are available, there is a consensus that parent-compound-specific assays correlate best with clinical events. Of these, HPLC is the standard reference, but the monoclonal antibody RIAs and the monoclonal antibody FPIA offer sensitivity, reproducibility, and convenience. Most clinicians base their monitoring on trough cyclosporine concentrations. Applied Pharmacokinetics, Principles of Therapeutic Drug Monitoring (1992) contains a broad discussion of cyclosporine pharmacokinetics and drug monitoring techniques. Blood concentration monitoring is not a replacement for renal function monitoring or tissue biopsies.
-
HOW SUPPLIED
Cyclosporine Capsules, USP [MODIFIED]
25 mg — Off-white, oval, soft gelatin capsules.
Imprinted in red: PA09
Packages of 30 unit-dose blisters.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C
(59° to 86°F) [See USP Controlled Room Temperature]100 mg — Off-white, oblong, soft gelatin capsules.
Imprinted in red: PA20
Packages of 30 unit-dose blisters.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C
(59° to 86°F) [See USP Controlled Room Temperature]Cyclosporine Oral Solution, USP [MODIFIED]
A light yellow liquid supplied in 50 mL bottles containing 100 mg/mL and a 4 mL dosing syringe with case.
Store and Dispense
In the original container at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Do not store in the refrigerator. Once opened, the contents must be used within two months. At temperatures below 68°F (20°C) the solution may gel; light flocculation or the formation of a light sediment or flakes may also form. Allow contents to reach room temperature to reverse these effects. There is no impact on product performance or dose.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 25 mg Capsule Blister Pack Box
NDC 75907-047-30
CycloSPORINE
Capsules, USP
[MODIFIED]25 mg
WARNING: CycloSPORINE
capsules, USP [MODIFIED] is
NOT BIOEQUIVALENT to
Sandimmune®* (CycloSPORINE
capsules, USP [NON-MODIFIED]).
Do NOT use interchangeably
without a physician's supervision.*Sandimmune® is a registered trademark of
Novartis Pharmaceuticals Corporation.Rx Only
30 Soft Gelatin CapsulesDr. Reddys's Laboratories, Inc
-
PRINCIPAL DISPLAY PANEL - 100 mg Capsule Blister Pack Box
NDC 75907-048-30
CycloSPORINE
Capsules, USP
[MODIFIED]100 mg
WARNING: CycloSPORINE capsules, USP
[MODIFIED] is NOT BIOEQUIVALENT to
Sandimmune®* (CycloSPORINE capsules, USP
[NON-MODIFIED]). Do NOT use interchangeably
without a physician's supervision.*Sandimmune® is a registered trademark of
Novartis Pharmaceuticals Corporation.Rx Only
30 Soft Gelatin CapsulesDr. Reddy's Laboratories, Inc
-
INGREDIENTS AND APPEARANCE
CYCLOSPORINE
cyclosporine capsule, liquid filledProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75907-047 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (CYCLOSPORINE - UNII:83HN0GTJ6D) CYCLOSPORINE 25 mg Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) POLYOXYL 35 CASTOR OIL (UNII: 6D4M1DAL6O) FERRIC OXIDE RED (UNII: 1K09F3G675) SHELLAC (UNII: 46N107B71O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) SORBITOL (UNII: 506T60A25R) CAPRYLOCAPROYL POLYOXYLGLYCERIDES 8 (UNII: 00BT03FSO2) Product Characteristics Color white (off white) Score no score Shape OVAL Size 9mm Flavor Imprint Code PA09 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75907-047-30 30 in 1 BOX 07/01/2024 1 NDC:75907-047-81 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065044 07/01/2024 CYCLOSPORINE
cyclosporine capsule, liquid filledProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75907-048 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSPORINE (UNII: 83HN0GTJ6D) (CYCLOSPORINE - UNII:83HN0GTJ6D) CYCLOSPORINE 100 mg Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) POLYOXYL 35 CASTOR OIL (UNII: 6D4M1DAL6O) FERRIC OXIDE RED (UNII: 1K09F3G675) SHELLAC (UNII: 46N107B71O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) SORBITOL (UNII: 506T60A25R) CAPRYLOCAPROYL POLYOXYLGLYCERIDES 8 (UNII: 00BT03FSO2) Product Characteristics Color white (off white) Score no score Shape OVAL (oblong) Size 22mm Flavor Imprint Code PA20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75907-048-30 30 in 1 BOX 07/01/2024 1 NDC:75907-048-81 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065044 07/01/2024 Labeler - Dr. Reddy's Laboratories Inc (802315887)