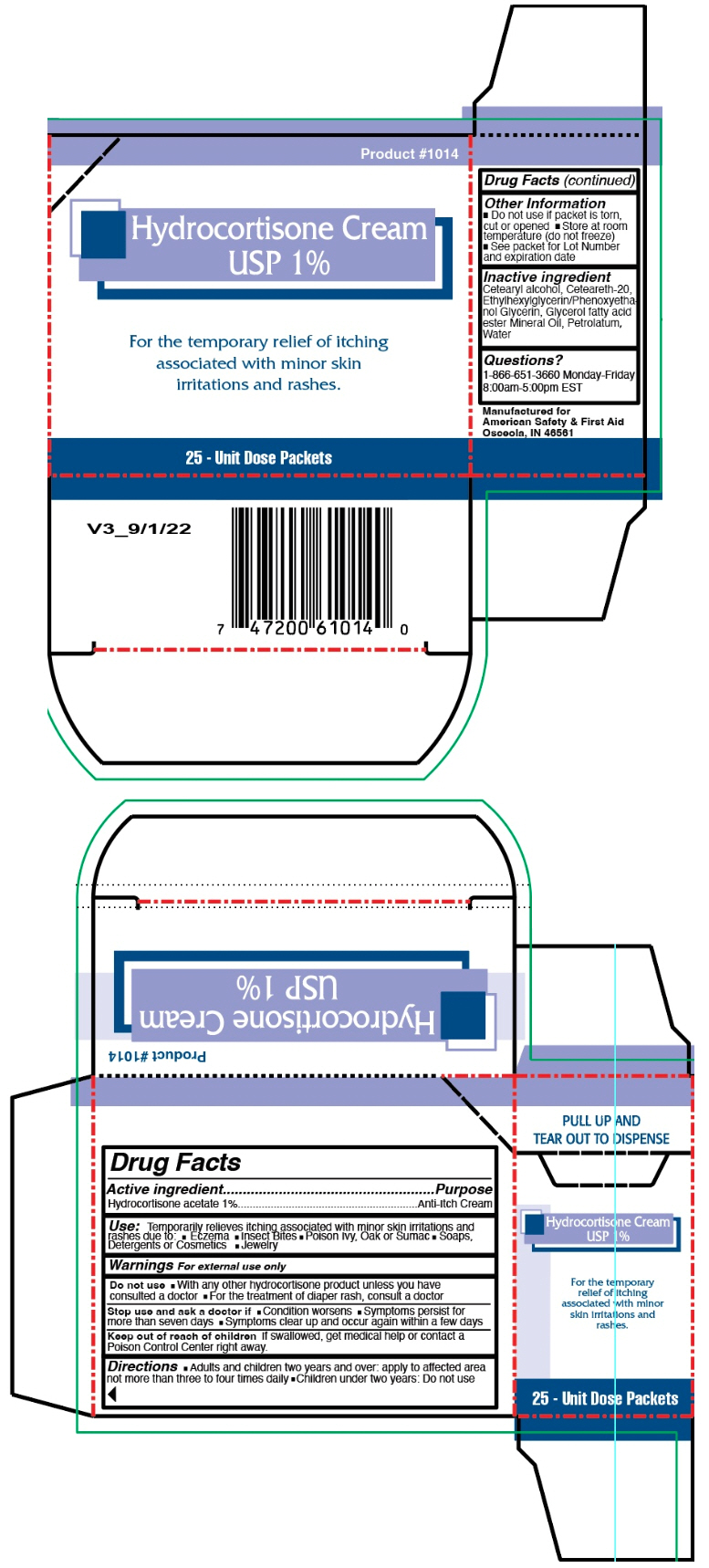
Label: HYDROCORTISONE- hydrocortisone acetate cream
- NDC Code(s): 73598-1014-1
- Packager: JHK Inc dba American Safety & First Aid
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Other Information
- Inactive ingredient
- Questions?
- PRINCIPAL DISPLAY PANEL - 25 Packet Carton
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE
hydrocortisone acetate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73598-1014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 0.9 g in 0.9 g Inactive Ingredients Ingredient Name Strength CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) PETROLATUM (UNII: 4T6H12BN9U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73598-1014-1 25 in 1 CARTON 02/02/2000 1 1 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 02/02/2000 Labeler - JHK Inc dba American Safety & First Aid (867236309)