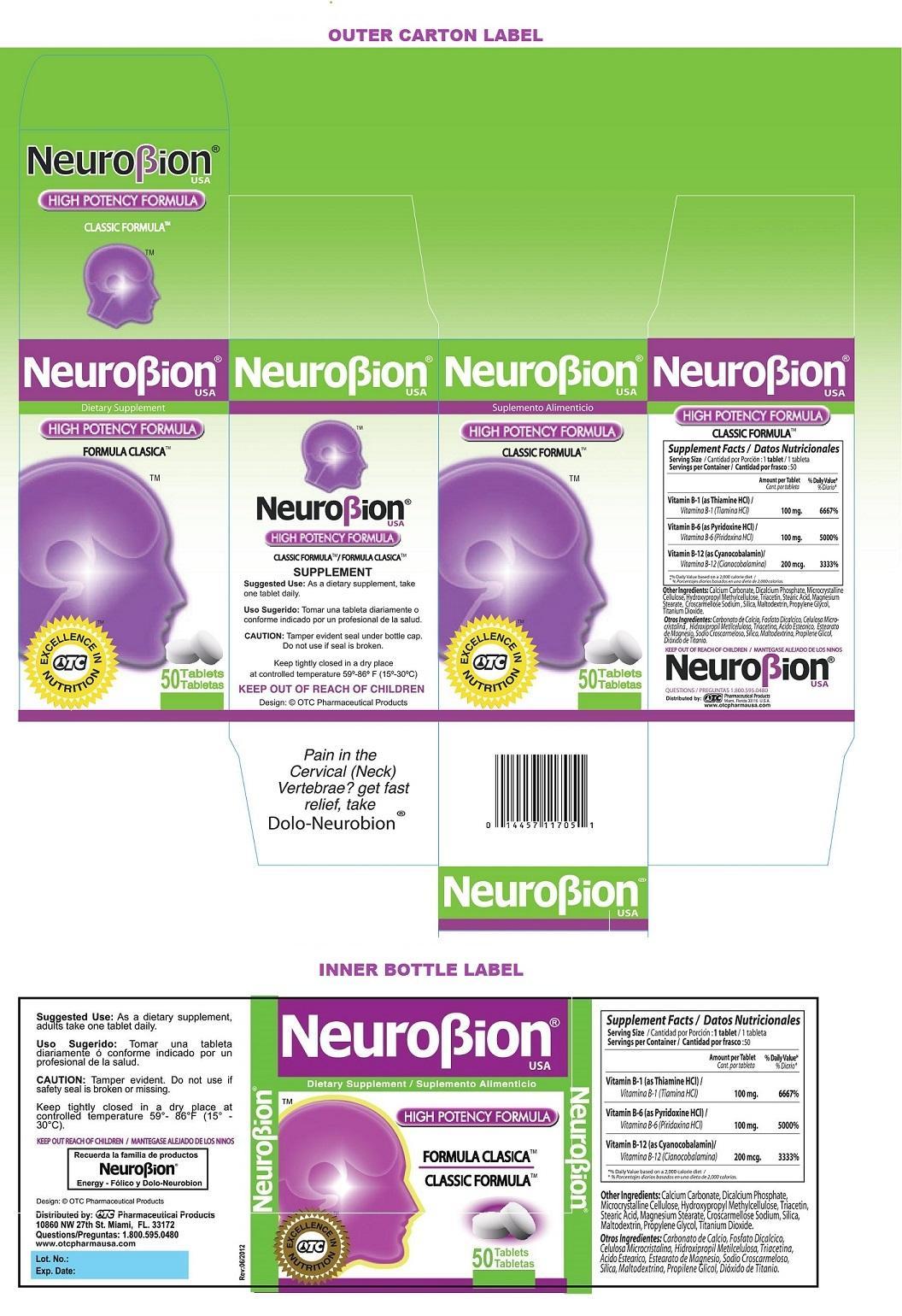
Label: NEUROBION- thiamine hydrochloride, pyridoxine hydrochloride, cyanocobalamin tablet
- NHRIC Code(s): 55959-117-05
- Packager: BENARD INDUSTRIES INC
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated October 30, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Dietary Supplement
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size : 1 tablet
Servings per Container : 50
Amount per Tablet % Daily Value* Vitamin B-1 (as Thiamine HCl) 100 mg. 6667% Vitamin B-6 (as Pyridoxine HCl) 100 mg. 5000% Vitamin B-12 (as Cyanocobalamin) 200 mcg. 3333% *% Daily value based on a 2,000 calorie diet Other Ingredients: Calcium Carbonate, Dicalcium Phosphate, Microcrystalline Cellulose, Hydroxypropyl Methylcellulose, Triacetin, Stearic Acid, Magnesium Stearate, Croscarmellose Sodium, Silica, Maltodextrin, Propylene Glycol, Titanium Dioxide.
- WARNINGS
- PRECAUTIONS
- SAFE HANDLING WARNING
- DOSAGE & ADMINISTRATION
- HEALTH CLAIM
- Packaging
-
INGREDIENTS AND APPEARANCE
NEUROBION
thiamine hydrochloride, pyridoxine hydrochloride, cyanocobalamin tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:55959-117 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 100 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 100 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 200 ug Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) TRIACETIN (UNII: XHX3C3X673) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MALTODEXTRIN (UNII: 7CVR7L4A2D) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:55959-117-05 1 in 1 CARTON 1 50 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 02/15/1984 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 11 mm scoring 1 imprint Labeler - BENARD INDUSTRIES INC (106700321) Establishment Name Address ID/FEI Business Operations Pharbest Pharmaceuticals, Inc. 557054835 manufacture(55959-117)