Label: UV PROTECT FACE SPF 50- zinc oxide, titanium dioxide cream
- NDC Code(s): 14324-011-00
- Packager: Naos USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
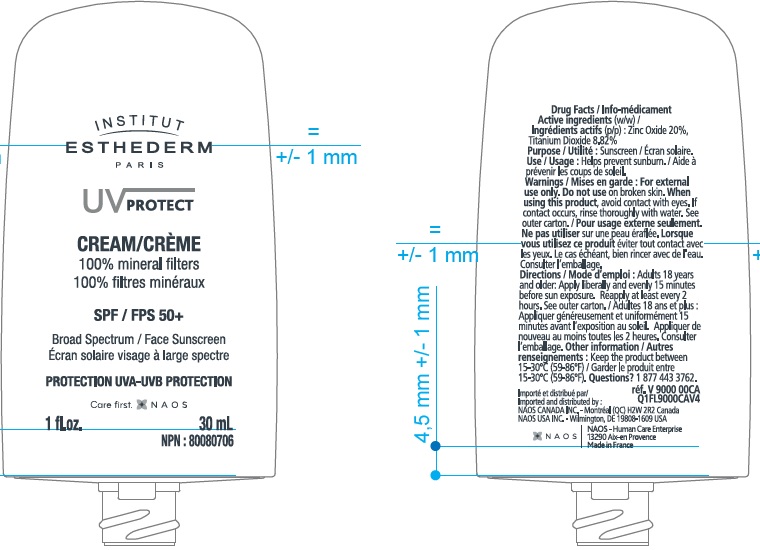
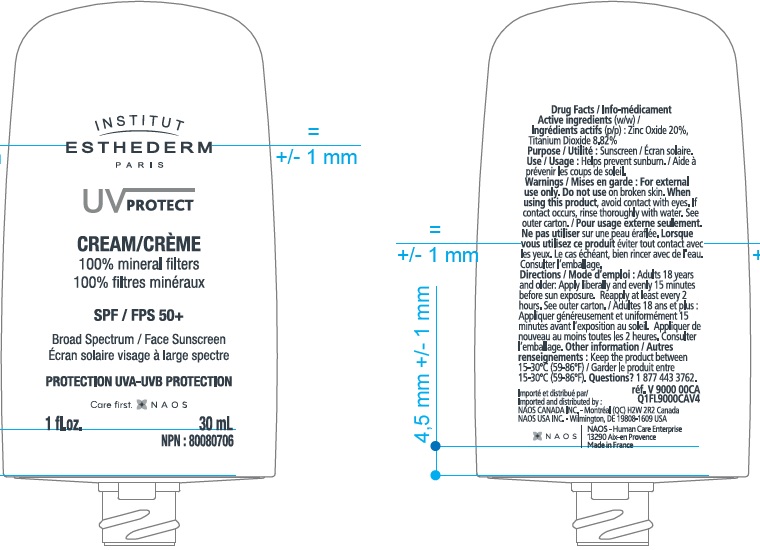
- Active Ingredients
- Uses
- Warnings
-
Directions
Adults 18 years and older:
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeve shirts, pants, hats and sunglasses.
- Children under 6 months of age: Ask a doctor.
- Other information
-
Inactive Ingredients
Coco-caprylate/caprate, Caprylic/Capric Triglyceride, Methyl Methacrylate Crosspolymer, Dicaprylyl Ether, Dicaprylyl Carbonate, Silica Dimethyl Silylate, Oryza Sativa (Rice) Bran Oil, Polyhydroxystearic Acid, Tocopheryl Acetate, Stearic Acid, Alumina, Fragrance (Parfum), Stearyl Glycyrrhetinate, Tocopherol, Farnesol.
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
UV PROTECT FACE SPF 50
zinc oxide, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14324-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 200 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 88.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DICAPRYLYL ETHER (UNII: 77JZM5516Z) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) WHITE RICE (UNII: A195V20H7A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) TOCOPHEROL (UNII: R0ZB2556P8) FARNESOL (UNII: EB41QIU6JL) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14324-011-00 1 in 1 BOX 01/31/2018 09/30/2025 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/31/2018 09/30/2025 Labeler - Naos USA, Inc. (080727572)