Label: THYROVET EQUINE- levothyroxine sodium powder
- NDC Code(s): 13985-575-01, 13985-575-10
- Packager: MWI/Vet One
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- VETERINARY INDICATIONS
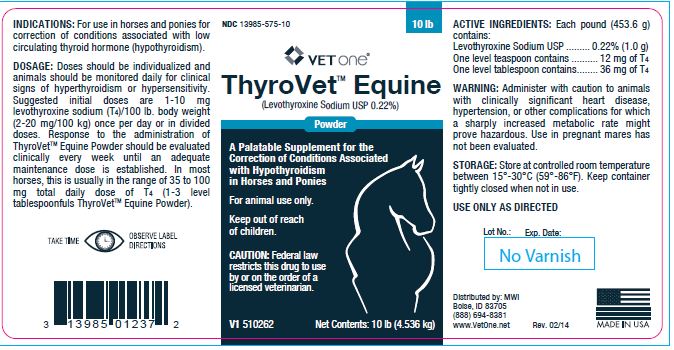
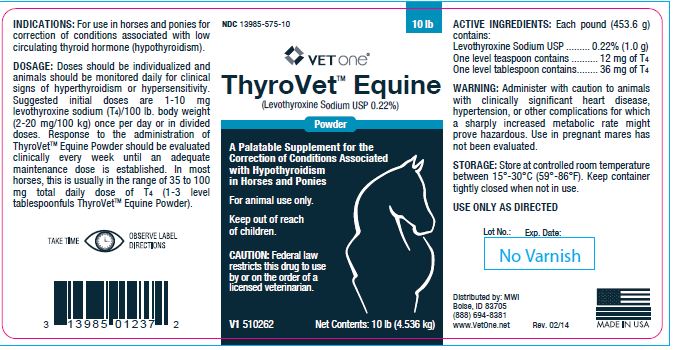
- INDICATIONS:
-
DOSAGE:
Doses should be individualized and animals should be monitored daily for clinical signs of hyperthyroidism or hypersensitivity. Suggested initial doses are 1-10 mg levothyroxine sodium (T4)/100 lb. body weight (2-20 mg/100 kg) once per day or in divided doses. Response to the administration of ThyroVetTM Equine Powder should be evaluated clinically every week until an adequate maintenance dose is established. In most horses, this is usually in the range of 35 to 100 mg total daily dose of T4 (1-3 level tablespoonfuls ThyroVetTM Equine Powder).
- ACTIVE INGREDIENTS:
- WARNING:
- STORAGE:
- Net Contents:
- INFORMATION FOR OWNERS/CAREGIVERS
- 1 lb (453.6 g)
- 10 lb (4.536 kg)
-
INGREDIENTS AND APPEARANCE
THYROVET EQUINE
levothyroxine sodium powderProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13985-575 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOTHYROXINE SODIUM (UNII: 9J765S329G) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 1000 mg in 453.6 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-575-01 12 in 1 CASE 1 453.6 g in 1 JAR 2 NDC:13985-575-10 1 in 1 CASE 2 453600 g in 1 PAIL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/17/2014 Labeler - MWI/Vet One (019926120) Registrant - First Priority Incorporated (179925722) Establishment Name Address ID/FEI Business Operations First Priority Incorporated 179925722 manufacture