Label: CHILDRENS LORATADINE- loratadine tablet, chewable
- NDC Code(s): 69842-954-30
- Packager: CVS PHARMACY, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 3, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
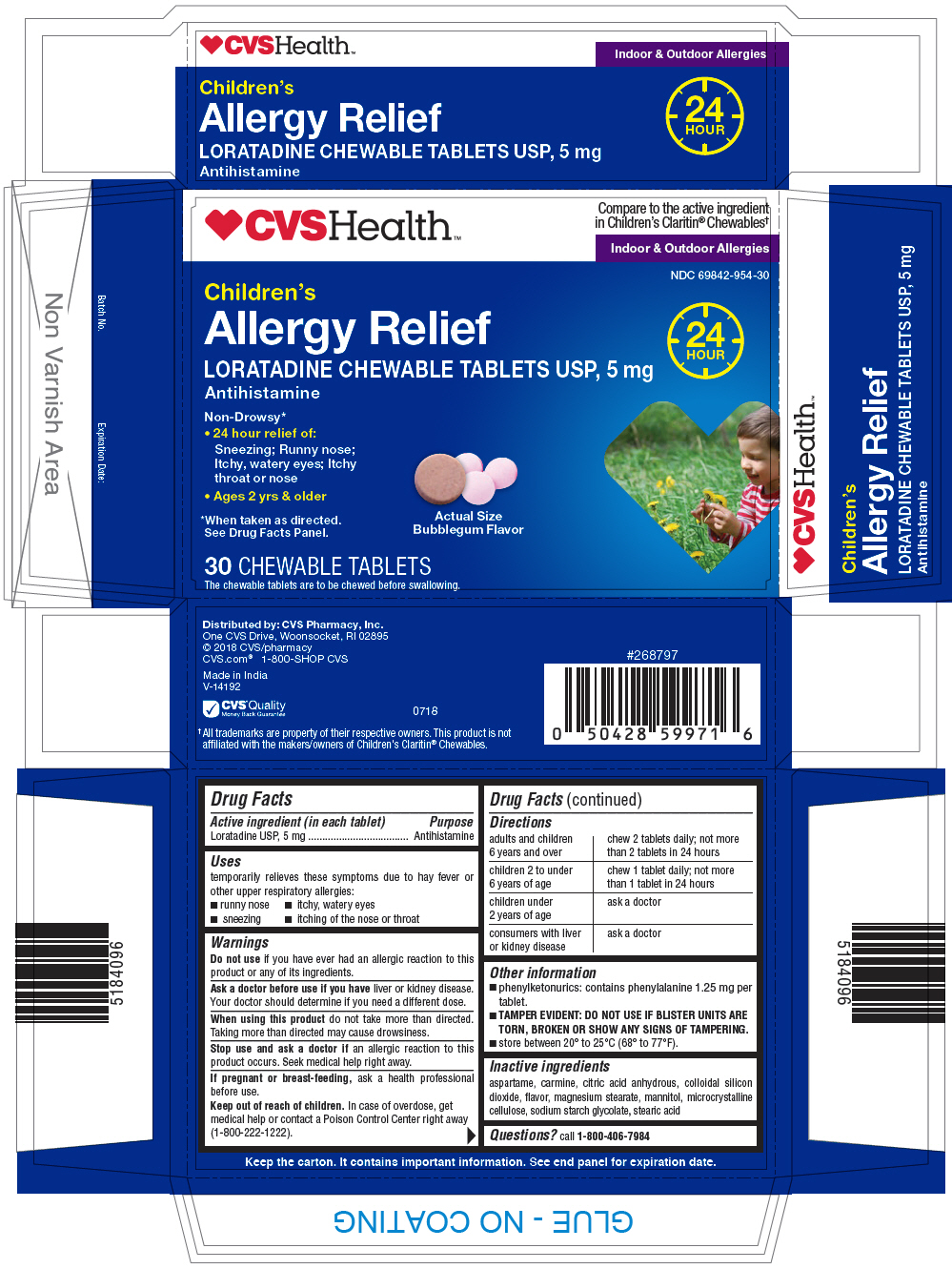
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Blister Pack Carton
CVS Health™
Compare to the active ingredient
in Children's Claritin® Chewables†Indoor & Outdoor Allergies
NDC 69842-954-30
Children's
Allergy ReliefLORATADINE CHEWABLE TABLETS USP, 5 mg
Antihistamine24
HOURNon-Drowsy*
- 24 hour relief of:
Sneezing; Runny nose;
Itchy, watery eyes; Itchy
throat or nose - Ages 2 yrs & older
*When taken as directed.
See Drug Facts Panel.Actual Size
Bubblegum Flavor30 CHEWABLE TABLETS
The chewable tablets are to be chewed before swallowing.
- 24 hour relief of:
-
INGREDIENTS AND APPEARANCE
CHILDRENS LORATADINE
loratadine tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-954 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 5 mg Inactive Ingredients Ingredient Name Strength aspartame (UNII: Z0H242BBR1) cochineal (UNII: TZ8Z31B35M) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) mannitol (UNII: 3OWL53L36A) microcrystalline cellulose (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) stearic acid (UNII: 4ELV7Z65AP) Product Characteristics Color pink (light to medium pink with a slightly speckled appearance) Score no score Shape ROUND (Flat face, beveled edge) Size 10mm Flavor BUBBLE GUM Imprint Code 754 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-954-30 3 in 1 CARTON 07/01/2018 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210088 07/01/2018 Labeler - CVS PHARMACY, INC (062312574) Registrant - Ohm Laboratories (051565745) Establishment Name Address ID/FEI Business Operations Ohm Laboratories 184769029 MANUFACTURE(69842-954)