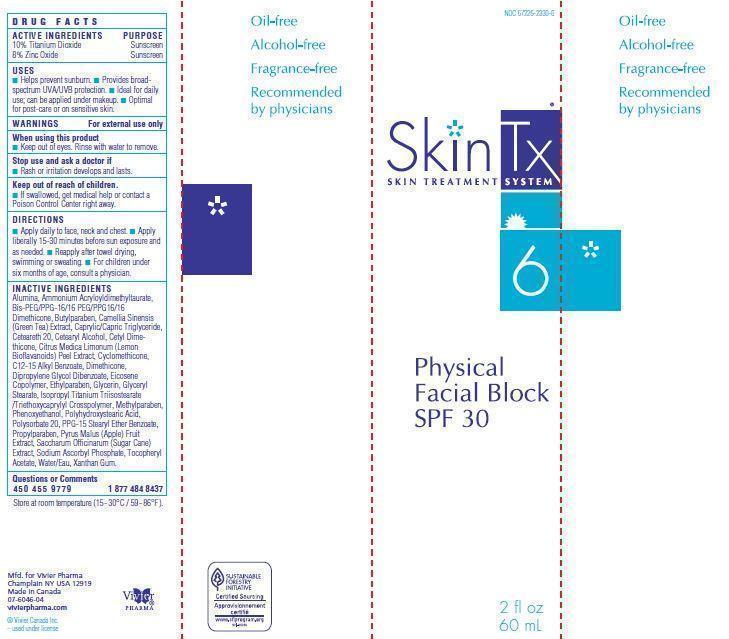
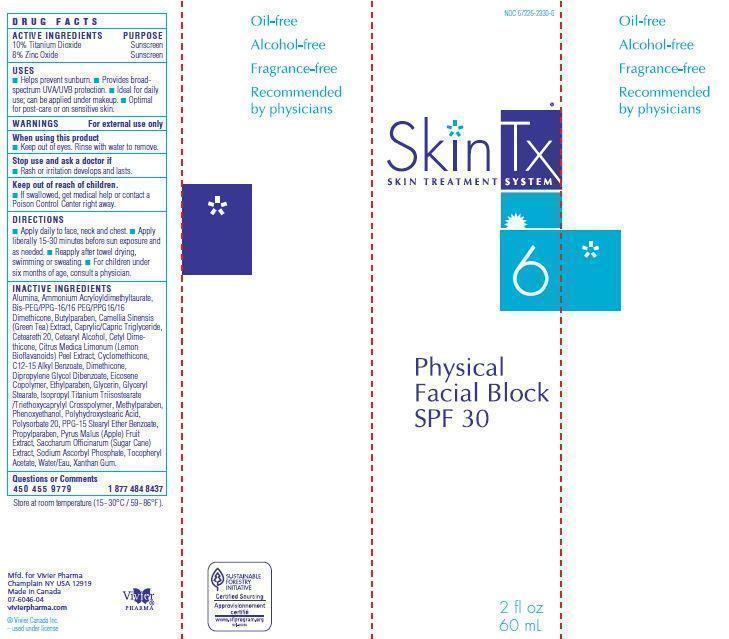
Label: PHYSICAL FACIAL BLOCK SPF 30- zinc oxide, titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 67226-2330-6 - Packager: Vivier Pharma, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 10, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS For external use only
- Active Ingredients
- Uses
-
Inactive Ingredients
Alumina, Ammonium Acryloyldimethyltaurate,
Bis-PEG/PPG-16/16 PEG/PPG16/16
Dimethicone, Butylparaben, Camellia Sinensis
(Green Tea) Extract, Caprylic/Capric Triglyceride,
Ceteareth 20, Cetearyl Alcohol, Cetyl Dimethicone,
Citrus Medica Limonum (Lemon
Bioflavanoids) Peel Extract, Cyclomethicone,
C12-15 Alkyl Benzoate, Dimethicone,
Dipropylene Glycol Dibenzoate, Eicosene
Copolymer, Ethylparaben, Glycerin, Glyceryl
Stearate, Isopropyl Titanium Triisostearate
/Triethoxycaprylyl Crosspolymer, Methylparaben,
Phenoxyethanol, Polyhydroxystearic Acid,
Polysorbate 20, PPG-15 Stearyl Ether Benzoate,
Propylparaben, Pyrus Malus (Apple) Fruit
Extract, Saccharum Officinarum (Sugar Cane)
Extract, Sodium Ascorbyl Phosphate, Tocopheryl
Acetate, Water/Eau, Xanthan Gum. - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PHYSICAL FACIAL BLOCK SPF 30
zinc oxide, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67226-2330 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 4.8 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 6 g in 100 mL Inactive Ingredients Ingredient Name Strength ALUMINUM OXIDE (UNII: LMI26O6933) AMMONIUM ACRYLOYLDIMETHYLTAURATE (UNII: KBC00G95HI) BUTYLPARABEN (UNII: 3QPI1U3FV8) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CYCLOMETHICONE (UNII: NMQ347994Z) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE (UNII: 92RU3N3Y1O) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) ETHYLPARABEN (UNII: 14255EXE39) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYSORBATE 20 (UNII: 7T1F30V5YH) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SUGARCANE (UNII: 81H2R5AOH3) APPLE (UNII: B423VGH5S9) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) BIS-PEG/PPG-16/16 PEG/PPG-16/16 DIMETHICONE (UNII: 55A74AJ3KB) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) CITRUS BIOFLAVONOIDS (UNII: BD70459I50) EICOSYL POVIDONE (UNII: XQQ9MKE2BJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67226-2330-6 1 in 1 BOX 1 60 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 08/28/2006 Labeler - Vivier Pharma, Inc. (250996550) Establishment Name Address ID/FEI Business Operations Vivier Pharma, Inc. 250996550 manufacture(67226-2330)