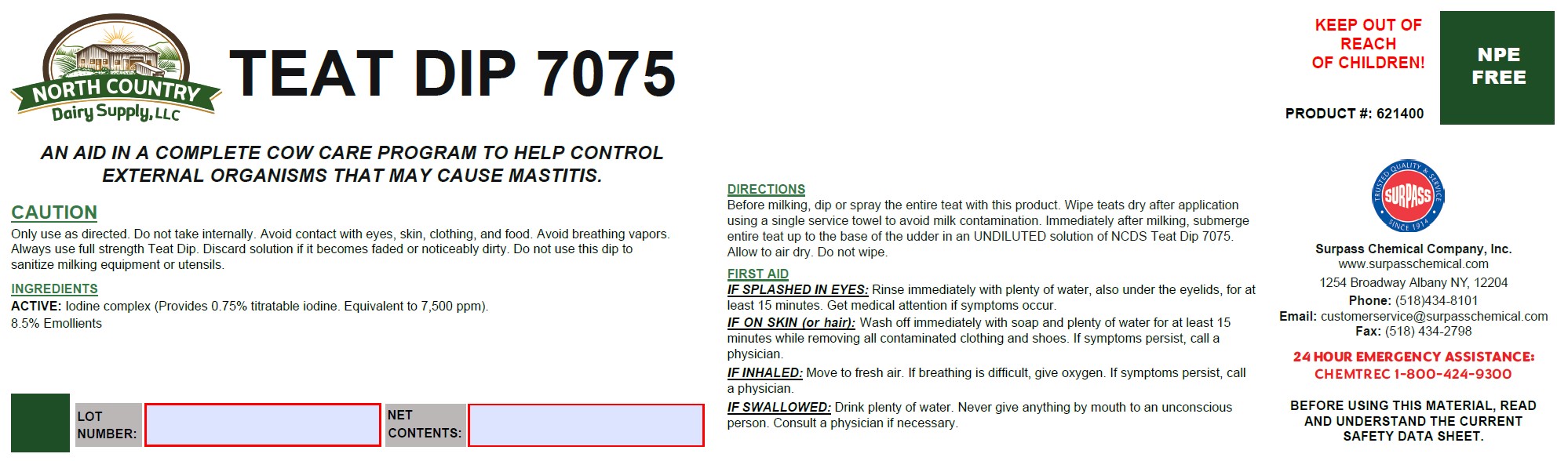
Label: TEAT DIP 7075- iodine solution
- NDC Code(s): 86067-0114-1, 86067-0114-2, 86067-0114-3, 86067-0114-4
- Packager: Surpass Chemical Company, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
INSTRUCTIONS FOR USE
DIRECTIONS:
Before milking, dip or spray the entire teat with this product. Wipe teats dry after application using a single service towel to avoid milk contamination. Immediately after milking, submerge entire teat up to the base of the udder in an UNDILUTED solution of a NCDS TD7075. Allow to air dry. Do not wipe. - GENERAL PRECAUTIONS
- ACTIVE INGREDIENT
-
OTHER SAFETY INFORMATION
FIRST AID:
IF SPLASHED IN EYES: Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. Get medical attention if symptoms occur.
IF ON SKIN (or hair): Wash off immediately with soap and plenty of water for at least 15 minutes while removing all contaminated clothing and shoes. If symptoms persist, call a physician.
IF INHALED: Move to fresh air. If breathing is difficult, give oxygen. If symptoms persist, call a physician.
IF SWALLOWED: Drink plenty of water. Never give anything by mouth to an unconscious person. Consult a physician if necessary. - SPL UNCLASSIFIED SECTION
- KEEP OUT OF REACH OF CHILDREN
- SAFE HANDLING WARNING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TEAT DIP 7075
iodine solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86067-0114 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 0.0349 kg in 1 kg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 0.8376 kg in 1 kg C9-11 PARETH-8 (UNII: 80E6PSE1XL) 0.0375 kg in 1 kg GLYCERIN (UNII: PDC6A3C0OX) 0.085 kg in 1 kg TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 0.005 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86067-0114-1 19.50 kg in 1 JUG 2 NDC:86067-0114-2 58.97 kg in 1 DRUM 3 NDC:86067-0114-3 118.39 kg in 1 DRUM 4 NDC:86067-0114-4 216.82 kg in 1 DRUM Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/18/2016 Labeler - Surpass Chemical Company, Inc. (002075133) Establishment Name Address ID/FEI Business Operations Surpass Chemical Company, Inc. 002075133 manufacture, api manufacture