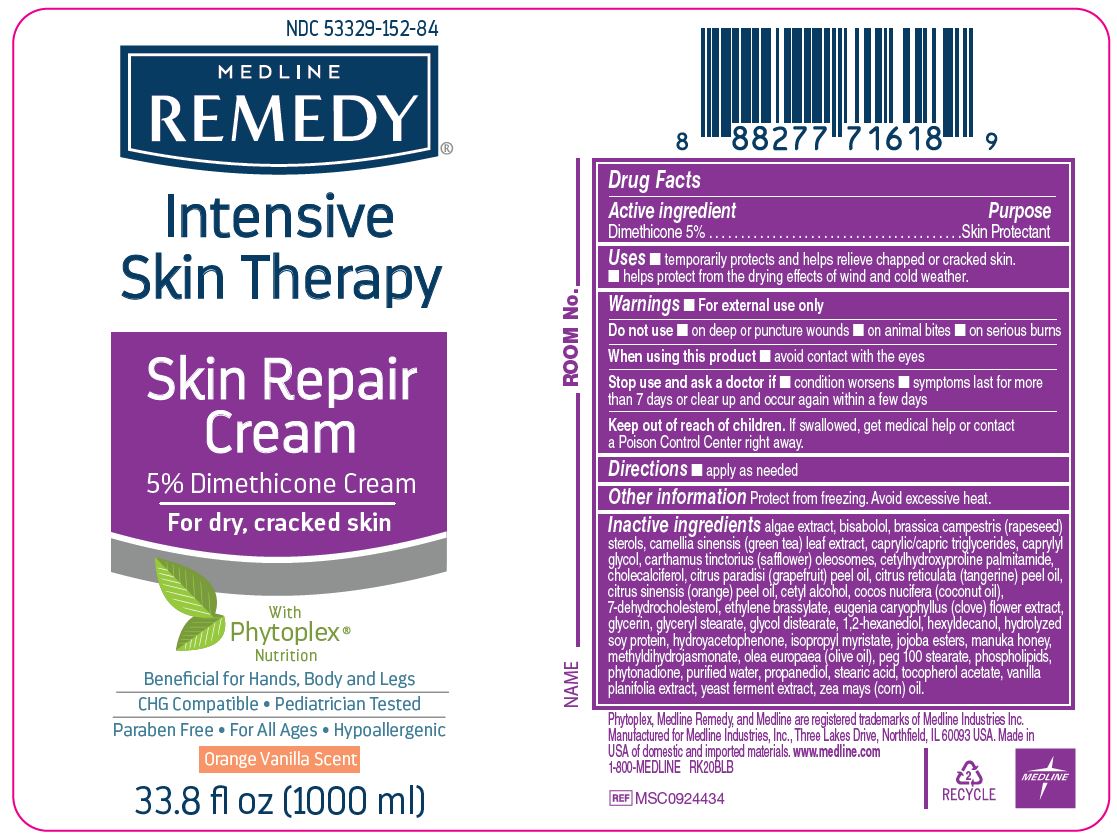
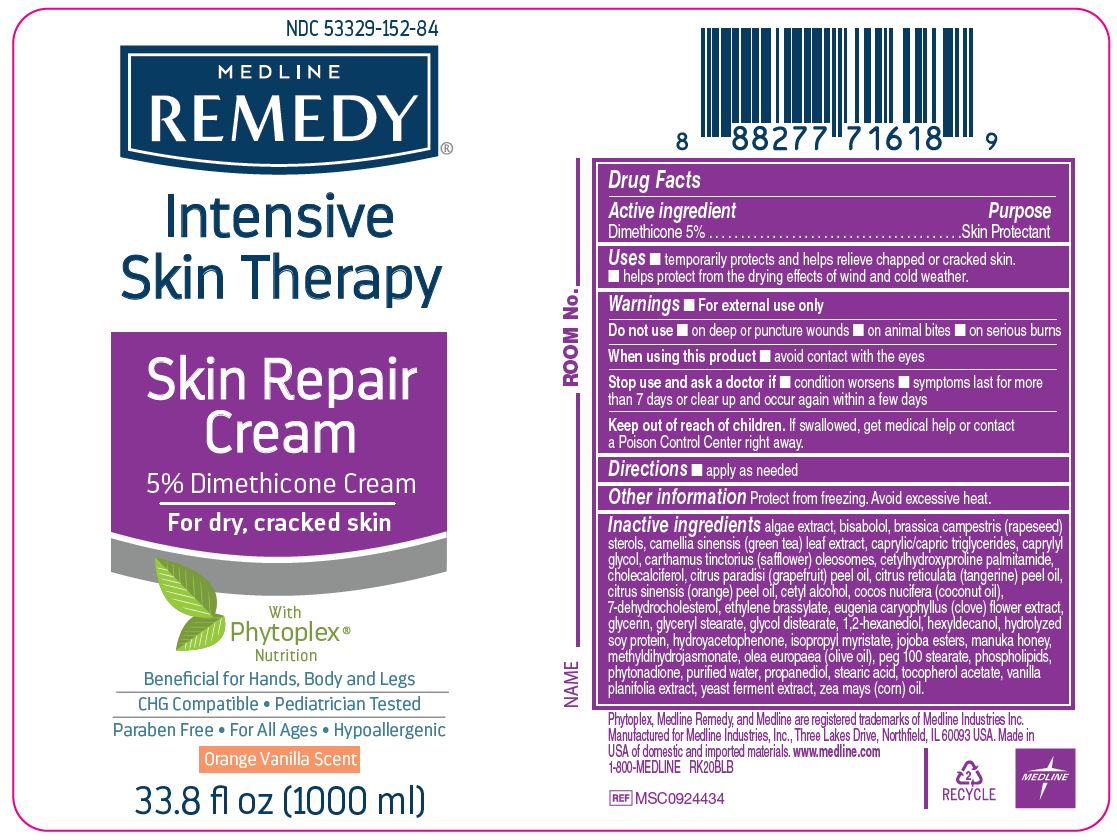
Label: MEDLINE REMEDY PHYTOPLEX SKIN REPAIR- dimethicone cream
-
NDC Code(s):
53329-152-04,
53329-152-14,
53329-152-17,
53329-152-44, view more53329-152-64, 53329-152-84
- Packager: Medline Industries, LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
algae extract, bisabolol, brassica campestris (rapeseed) sterols, camellia sinensis (green tea) leaf extract, caprylic/capric triglycerides, caprylyl glycol, carthamus tinctorius (safflower) oleosomes, cetylhydroxyproline palmitamide, cholecalciferol, citrus paradisi (grapefruit) peel oil, citrus reticulata (tangerine) peel oil, citrus sinensis (orange) peel oil, cetyl alcohol, cocos nucifera (coconut oil),
7-dehydrocholesterol, ethylene brassylate, eugenia caryophyllus (clove) flower extract, glycerin, glyceryl stearate, glycol distearate, 1,2-hexanediol, hexyldecanol, hydrolyzed soy protein, hydroyacetophenone, isopropyl myristate, jojoba esters, manuka honey, methyldihydrojasmonate, olea europaea (olive oil), peg 100 stearate, phospholipids, phytonadione, purified water, propanediol, stearic acid, tocopherol acetate, vanilla planifolia extract, yeast ferment extract, zea mays (corn) oil. - Manufacturing Information
- Package Label
-
INGREDIENTS AND APPEARANCE
MEDLINE REMEDY PHYTOPLEX SKIN REPAIR
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53329-152 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength GELIDIELLA ACEROSA (UNII: T91K54D6M1) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) CARTHAMUS TINCTORIUS (SAFFLOWER) OLEOSOMES (UNII: 9S60Q72309) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) HONEY (UNII: Y9H1V576FH) PHYTONADIONE (UNII: A034SE7857) ORANGE OIL (UNII: AKN3KSD11B) CLOVE (UNII: K48IKT5321) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CHOLECALCIFEROL (UNII: 1C6V77QF41) MANDARIN OIL (UNII: NJO720F72R) COCONUT OIL (UNII: Q9L0O73W7L) GRAPEFRUIT OIL (UNII: YR377U58W9) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PROPANEDIOL (UNII: 5965N8W85T) GLYCOL DISTEARATE (UNII: 13W7MDN21W) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYLDECANOL (UNII: 151Z7P1317) .BETA.-BISABOLOL (UNII: LP618AV2EA) OLIVE OIL (UNII: 6UYK2W1W1E) CETYLHYDROXYPROLINE PALMITAMIDE (UNII: 74ONU0S62G) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERIN (UNII: PDC6A3C0OX) RAPESEED STEROL (UNII: B46B6DD20U) STEARIC ACID (UNII: 4ELV7Z65AP) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) 7-DEHYDROCHOLESTEROL (UNII: BK1IU07GKF) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) VANILLIN (UNII: CHI530446X) WATER (UNII: 059QF0KO0R) CORN OIL (UNII: 8470G57WFM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53329-152-14 59 mL in 1 TUBE; Type 0: Not a Combination Product 02/12/2018 2 NDC:53329-152-44 118 mL in 1 TUBE; Type 0: Not a Combination Product 02/12/2018 3 NDC:53329-152-64 946.353 mL in 1 TUBE; Type 0: Not a Combination Product 04/03/2018 4 NDC:53329-152-17 144 in 1 BOX 04/03/2018 4 NDC:53329-152-04 4.14 mL in 1 PACKET; Type 0: Not a Combination Product 5 NDC:53329-152-84 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/12/2019 6 NDC:53329-152-04 4.14 mL in 1 PACKET; Type 0: Not a Combination Product 02/12/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M016 02/12/2018 Labeler - Medline Industries, LP (025460908) Registrant - Medline Industries, LP (025460908)