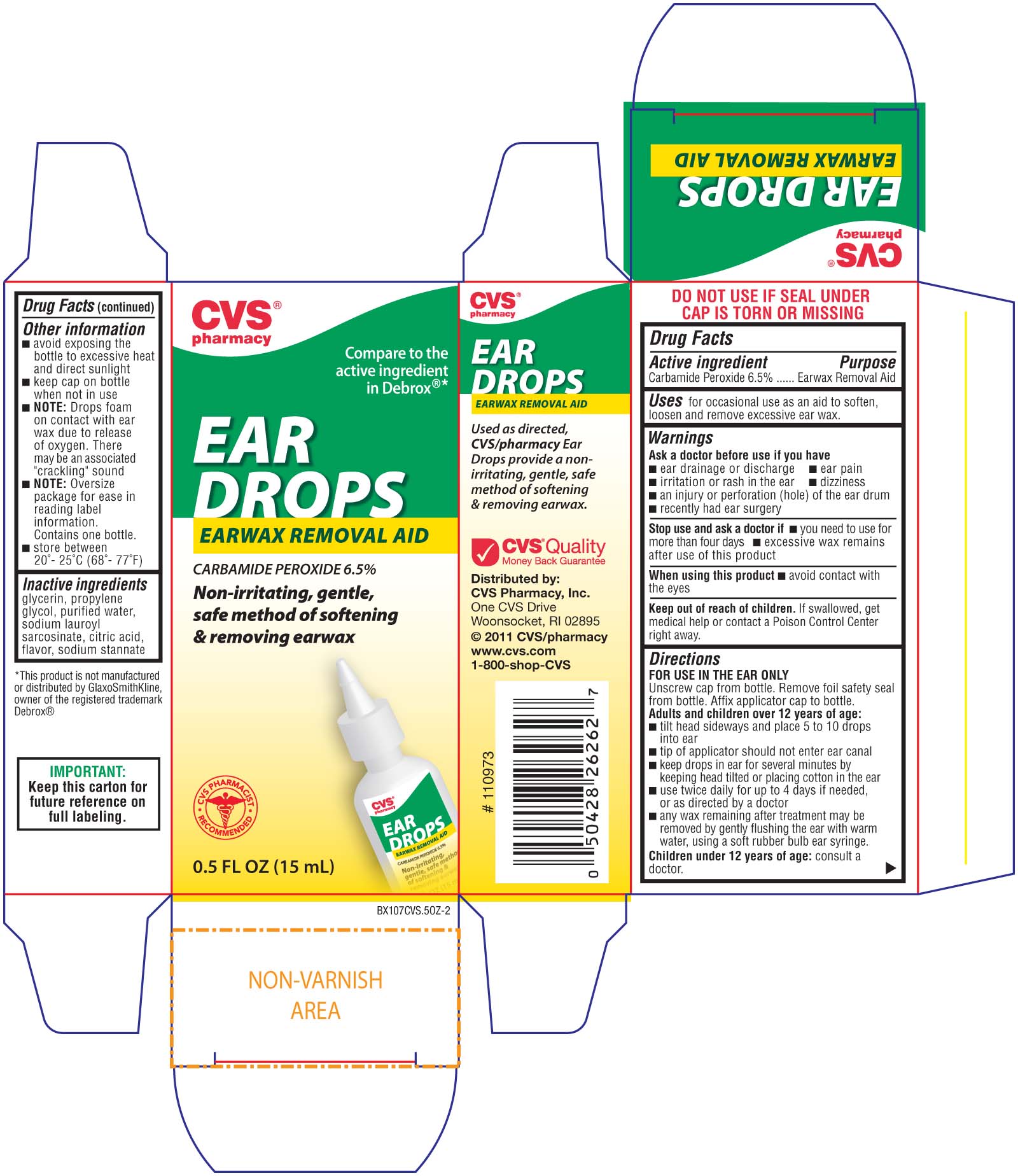
Label: CVS EARWAX REMOVAL AID- carbamide peroxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 59779-257-27 - Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 25, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Ask a doctor before use if you have
- ear drainage or discharge - ear pain
- irritation or rash in the ear - dizziness
- an injury or perforation (hole) of the ear drum
- recently had ear surgery
Stop use and ask a doctor if
- you need to use for more than four days
- excessive wax remains after use of this product
When using this product - avoid contact with the eyes
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away -
DOSAGE & ADMINISTRATION
Directions
FOR USE IN THE EAR ONLY
Unscrew cap from bottle. Remove foil safety seal from bottle. Affix applicator cap to bottle.
Adults and children over 12 years of age:
- tilt head sideways and place 5 to 10 drops into ear
- tip of applicator should not enter ear canal
- keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear
- use twice daily for up to 4 days if needed, or as directed by a doctor
- any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber ear syringe.
Children under 12 years of age: consult a doctor.
Other information
- avoid exposing the bottle to excessive heat and direct sunlight
- keep cap on bottle when not in use
- Note: Drops foam on contact with ear wax due to release of oxygen. There may be an associated "cracking" sound
- Note: Oversize packaging for ease in reading label information. Contains one bottle.
- Store between 20 degrees to 25 degrees C (68 degrees to 77 degrees F)
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS EARWAX REMOVAL AID
carbamide peroxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-257 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (CARBAMIDE PEROXIDE - UNII:31PZ2VAU81) CARBAMIDE PEROXIDE 65 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) SODIUM STANNATE (UNII: NJ7C1V83KG) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color Score Shape Size Flavor MINT (FLAVOR) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-257-27 1 in 1 CARTON 1 15 mL in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part344 04/25/2011 Labeler - CVS Pharmacy (062312574) Registrant - Pharma Pac, LLC (140807475) Establishment Name Address ID/FEI Business Operations Pharma Pac, LLC 140807475 manufacture