Label: WHITE MALLOW DIAPER RASH CREAM- zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 55946-702-65, 55946-702-66 - Packager: Weleda, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 26, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
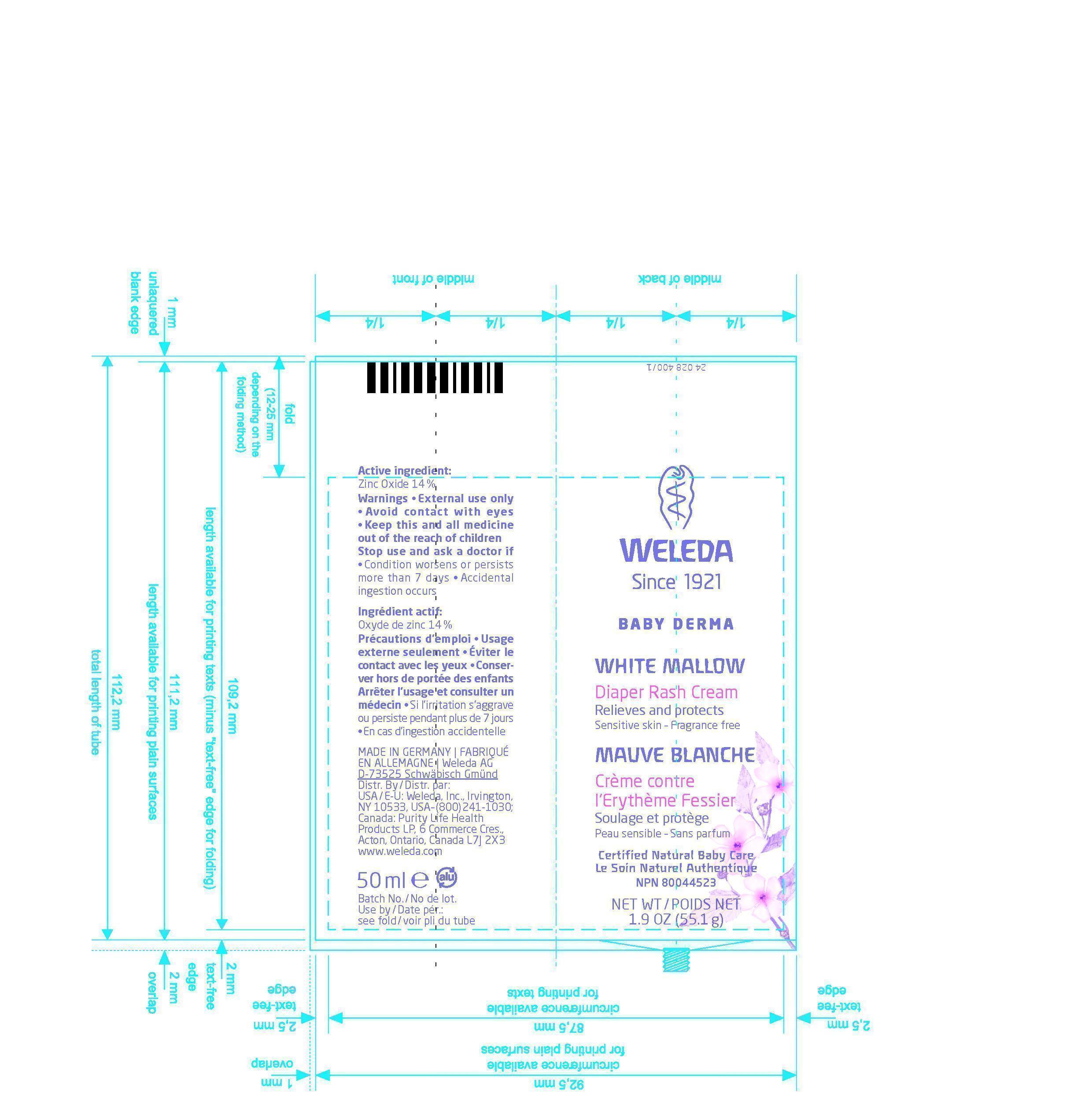
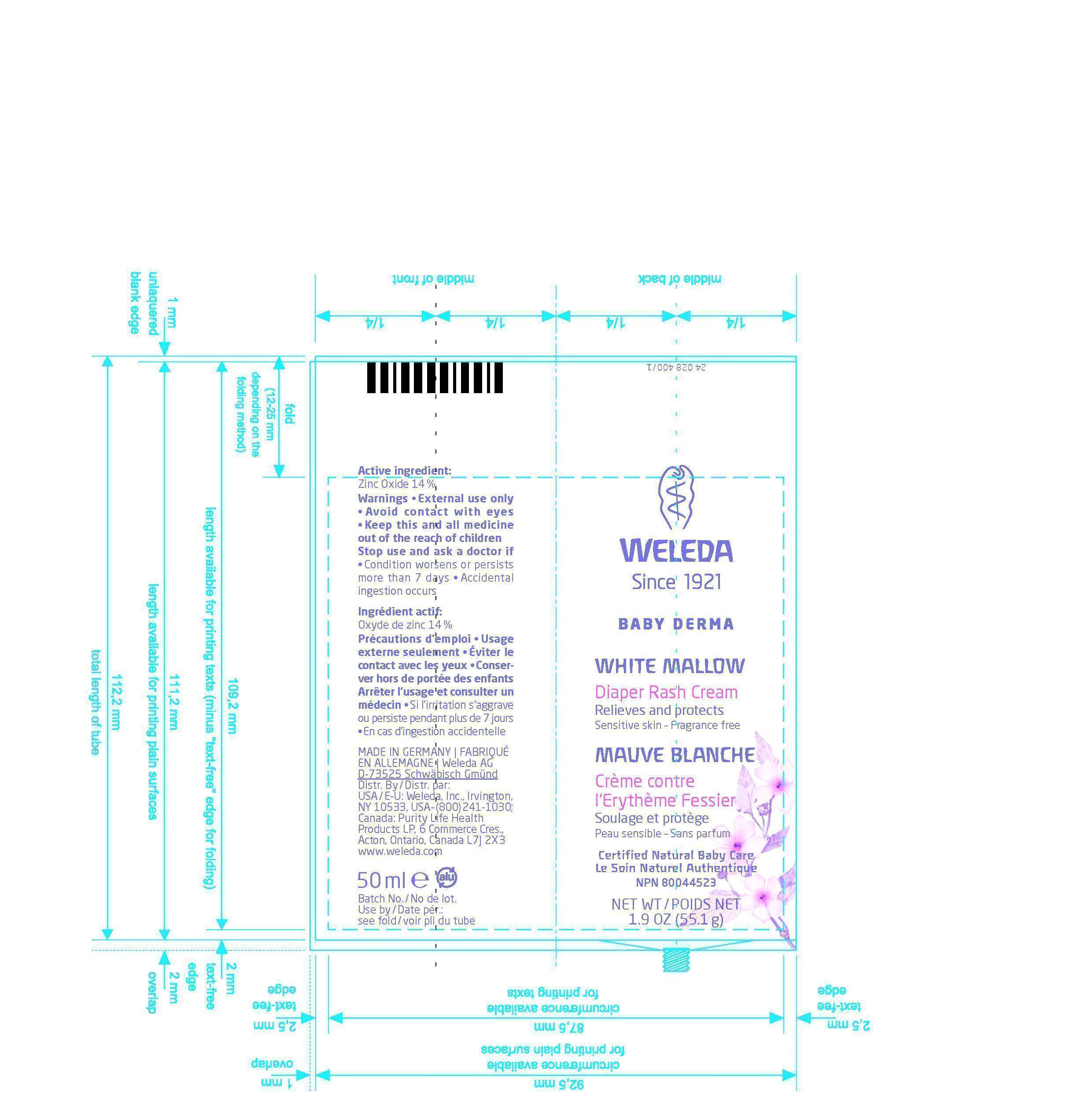
PRINCIPAL DISPLAY PANEL
Weleda
Since 1921
Baby Derma
White Mallow
Diaper Rash Cream
Relieves and protects
Sensitive Skin - Fragrance free
Mauve Blanche
Crème contre l`Erythème Fessier
Soulage et protège
Peau sensible - Sans parfum
Certified Natural Baby Care
Le Soin Naturel Authentique
NPN 80044523
NET WT / POIDS NET 1.9 OZ (55.1g)


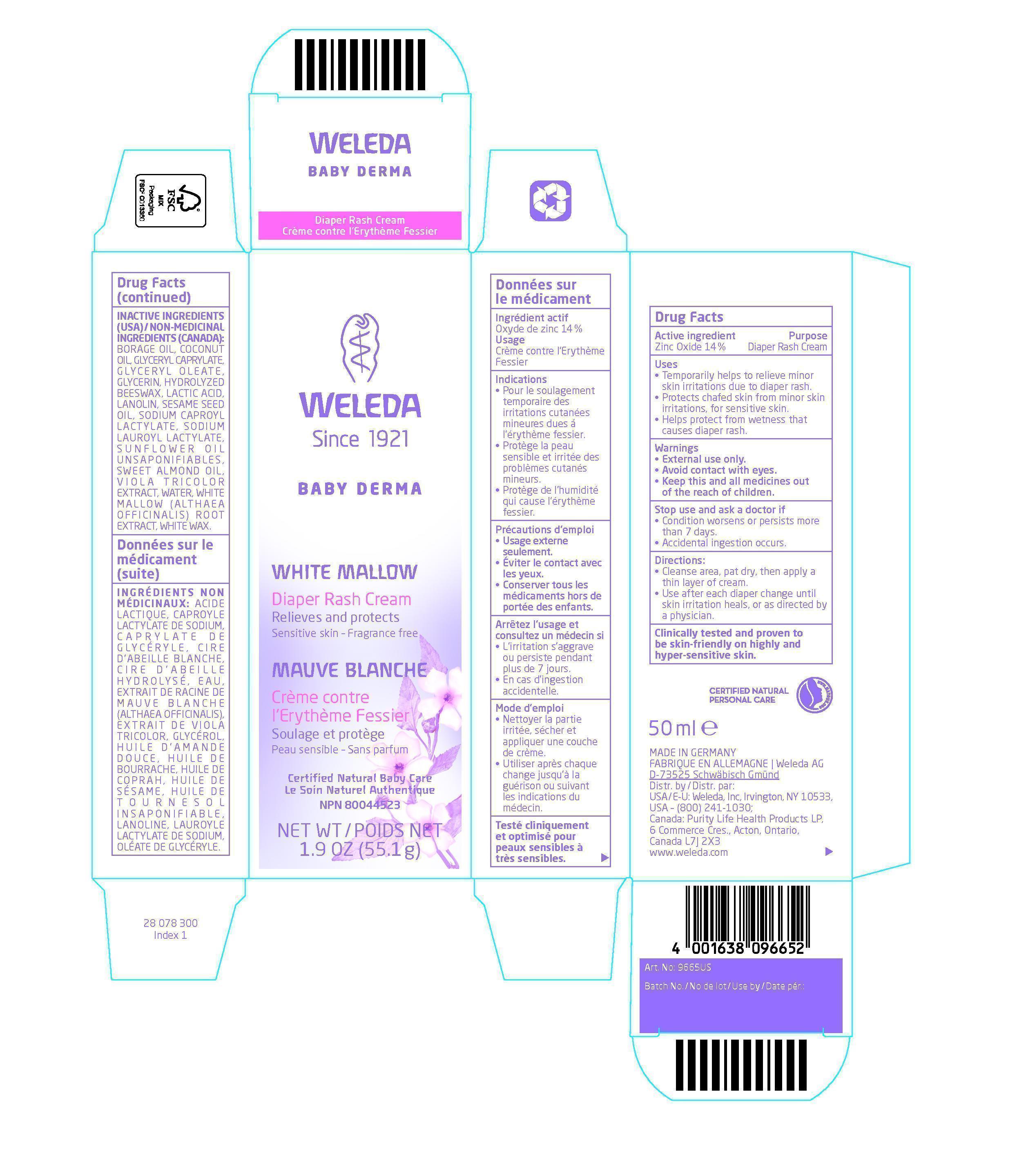
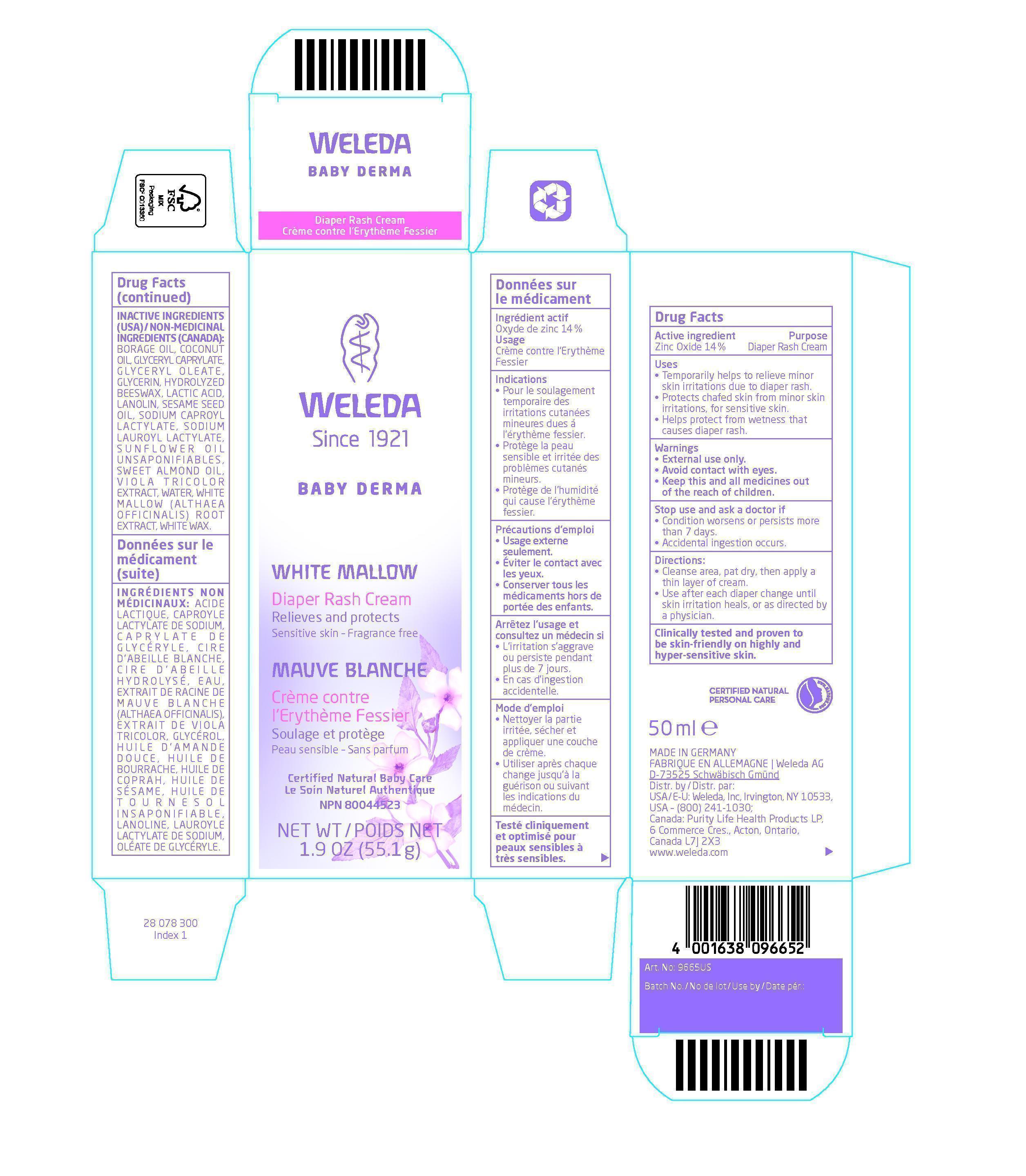
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients (USA) / Non-Medicinal Ingredients (Canada):
Borage Oil, Coconut Oil, Glyceryl Caprylate, Glyceryl Oleate, Glycerin, Hydrolyzed Beeswax, Lactic Acid, Lanolin, Sesame Seed Oil, Sodium Caproyl Lactylate, Sodium Lauroyl Lactylate, Sunflower Oil Unsaponifiables, Sweet Almond Oil, Viola tricolor Extract, Water, White Mallow (Althaea officinalis) Root Extract, White Wax.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WHITE MALLOW DIAPER RASH CREAM
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55946-702 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 14 g in 100 g Inactive Ingredients Ingredient Name Strength BORAGE OIL (UNII: F8XAG1755S) COCONUT OIL (UNII: Q9L0O73W7L) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) GLYCERYL OLEATE (UNII: 4PC054V79P) GLYCERIN (UNII: PDC6A3C0OX) YELLOW WAX (UNII: 2ZA36H0S2V) LACTIC ACID (UNII: 33X04XA5AT) LANOLIN (UNII: 7EV65EAW6H) SESAME OIL (UNII: QX10HYY4QV) SODIUM CAPROYL LACTYLATE (UNII: 87WR3BHC09) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) SUNFLOWER OIL (UNII: 3W1JG795YI) ALMOND OIL (UNII: 66YXD4DKO9) VIOLA TRICOLOR (UNII: 9Q24RAI43V) WATER (UNII: 059QF0KO0R) ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) WHITE WAX (UNII: 7G1J5DA97F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55946-702-65 55.1 g in 1 TUBE 2 NDC:55946-702-66 3 g in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 08/26/2014 Labeler - Weleda, Inc. (012301297) Registrant - Weleda, Inc. (012301297) Establishment Name Address ID/FEI Business Operations Weleda A.G. Schwäbisch Gmünd, Zweigniederlassung der Weled 315541862 manufacture(55946-702)