Label: PROFEND NASAL DECOLONIZATION- povidone iodine usp, 10% w/w swab
- NDC Code(s): 10819-3888-1, 10819-3888-2, 10819-3888-3
- Packager: Professional Disposables International, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 5, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
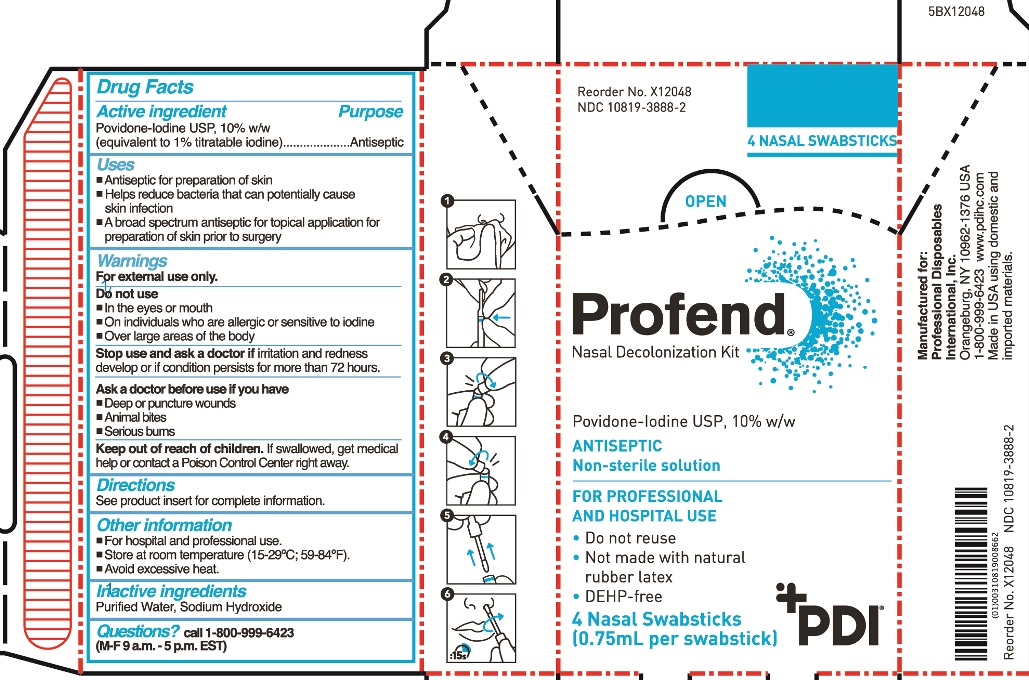
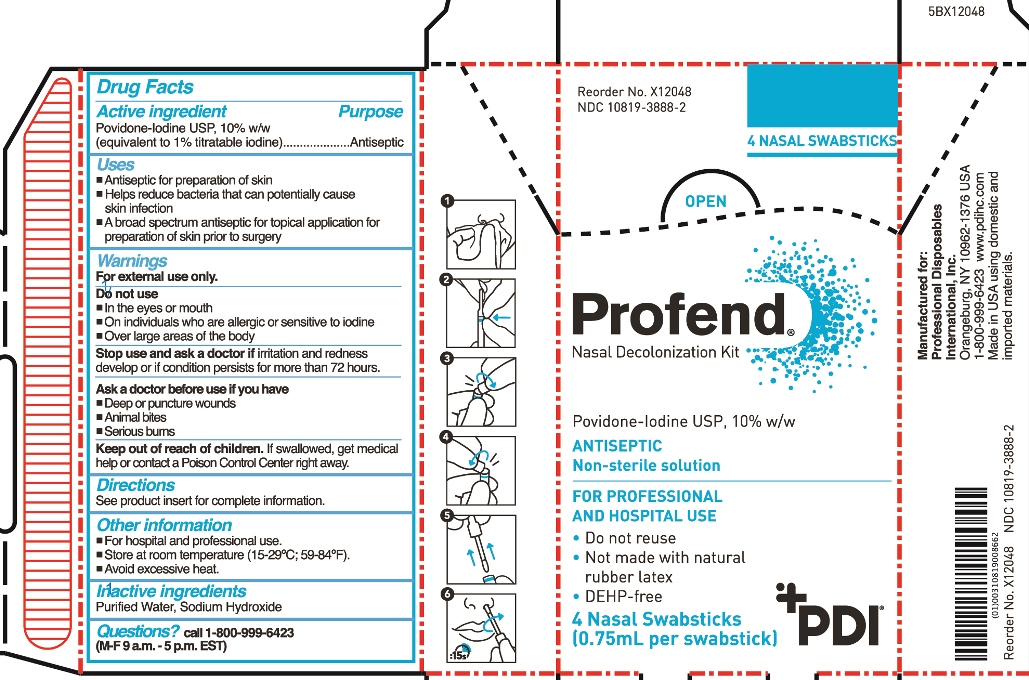
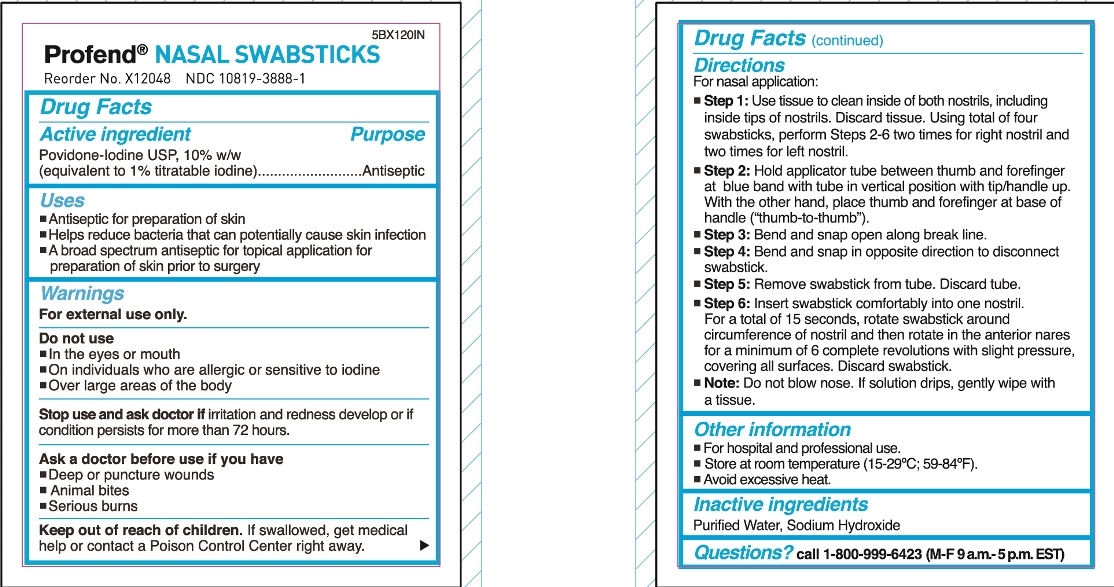
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- in the eyes or mouth
- on individuals who are allergic or sensitive to iodine
- over large areas of the body
Stop use and ask doctor if irritation and redness develop or if condition persists for more than 72 hours.
Ask a doctor before use if you have
- Deep or puncture wounds
- animal bites
- serious burns
- Keep out of reach of children.
-
Directions
For nasal application:
Step 1: Use tissue to clean inside of both nostrils, including inside tips of nostrils. Discard tissue.
Using total of four swabsticks, perform Steps 2-6 two times for right nostril and two times for left nostril.
Step 2: Hold applicator tube between thumb and forefinger at blue band with tube in vertical position with tip/handle up. With the other hand, place thumb and forefinger at base of handle (“thumb-to-thumb”).
Step 3: Bend and snap open along break line.
Step 4: Bend and snap in opposite direction to disconnect swabstick.
Step 5: Remove swabstick from tube. Discard tube.
Step 6: Insert swabstick comfortably into one nostril. For a total of 15 seconds, rotate swabstick around circumference of nostril and then rotate in the anterior nares for a minimum of 6 complete revolutions with slight pressure, covering all surfaces. Discard swabstick.
Note: Do not blow nose. If solution drips, gently wipe with a tissue.
- Other information
- Inactive Ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PROFEND NASAL DECOLONIZATION
povidone iodine usp, 10% w/w swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10819-3888 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10819-3888-3 12 in 1 CARTON 02/16/2018 1 NDC:10819-3888-2 4 in 1 BOX 1 NDC:10819-3888-1 0.75 mL in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 02/16/2018 Labeler - Professional Disposables International, Inc. (800777117) Establishment Name Address ID/FEI Business Operations Professional Disposables International, Inc. 800777117 manufacture(10819-3888)