Label: POLYFLEX- ampicillin injection, powder, for suspension
- NDC Code(s): 0010-4712-02
- Packager: Boehringer Ingelheim Animal Health USA Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated May 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION:
-
DESCRIPTION:
POLYFLEX (ampicillin for injectable suspension) is a broad-spectrum penicillin which has bactericidal activity against a wide range of common gram-positive and gram-negative bacteria.
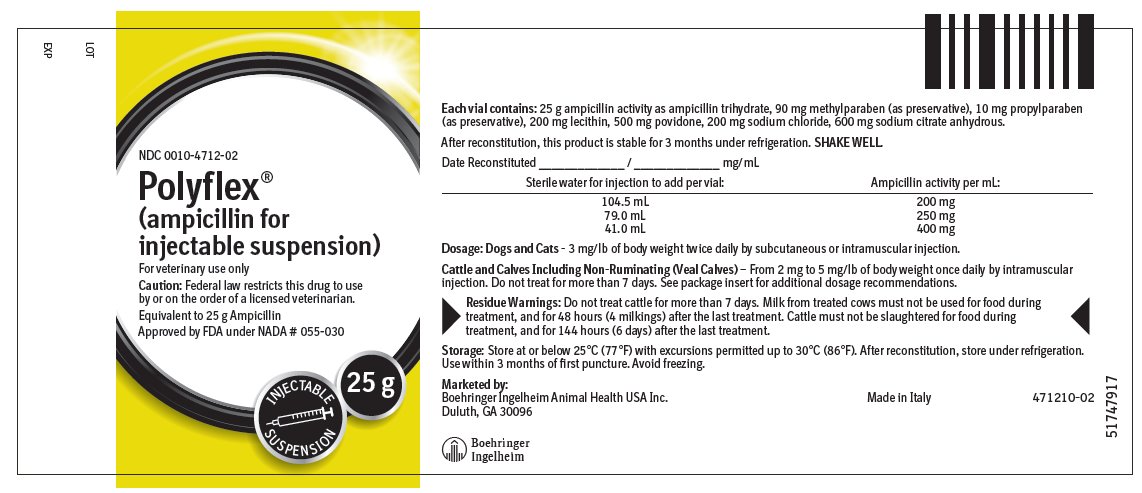
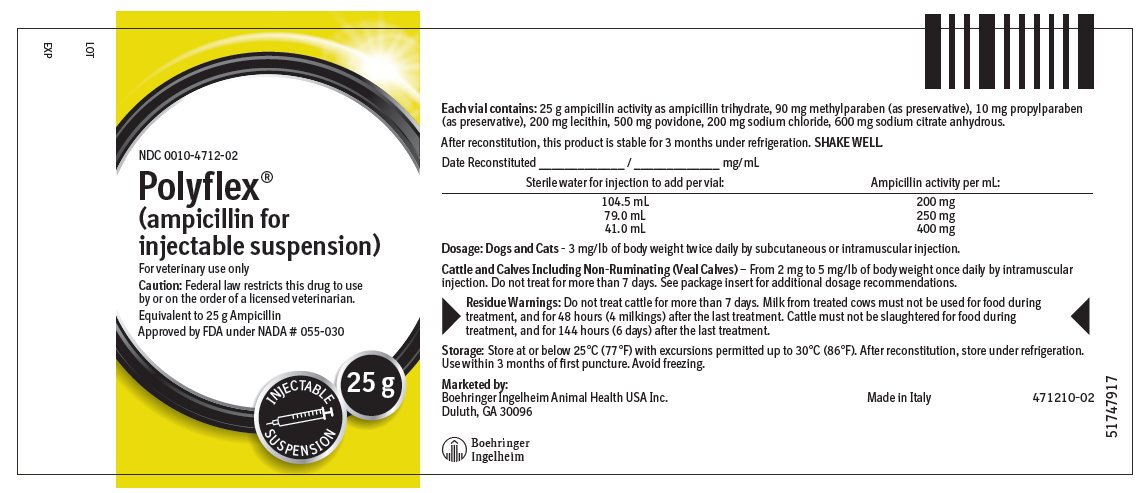
Each 25 g vial contains: 25 g ampicillin activity as ampicillin trihydrate, 90 mg methylparaben (as preservative), 10 mg propylparaben (as preservative), 200 mg lecithin, 500 mg povidone, 200 mg sodium chloride, 600 mg sodium citrate anhydrous.
-
INDICATIONS:
POLYFLEX has proved effective in the treatment of many infections previously beyond the spectrum of penicillin therapy. This drug is particularly indicated in the treatment of the following infections caused by susceptible strains of organisms:
Dogs and Cats — Respiratory Tract Infections: Upper respiratory infections, tonsillitis and bronchopneumonia due to hemolytic streptococci, Staphylococcus aureus, Escherichia coli,
Proteus mirabilis and Pasteurella spp.
Urinary Tract Infections due to Proteus mirabilis, Escherichia coli, Staphylococcus spp., hemolytic streptococci and Enterococcus spp.
Gastrointestinal Infections due to Enterococcus spp., Staphylococcus spp. and Escherichia coli.
Skin, Soft Tissue and Post-Surgical Infections: Abscesses, pustular dermatitis, cellulitis and infections of the anal gland, due to Escherichia coli, Proteus mirabilis, hemolytic streptococci, Staphylococcus spp. and Pasteurella spp.
Cattle and Calves Including Non-Ruminating (Veal Calves) — Respiratory Tract Infections:
Bacterial pneumonia (shipping fever, calf pneumonia and bovine pneumonia) caused by Aerobacter spp., Klebsiella spp., Staphylococcus spp., Streptococcus spp., Pasteurella multocida and E. coli susceptible to ampicillin trihydrate.
-
DOSAGE:
The dosage of POLYFLEX will vary according to the animal being treated, the severity of the infection and the animal’s response.
Dogs and Cats — The recommended dose for dogs or cats is 3 mg/lb of body weight administered twice daily by subcutaneous or intramuscular injection.
Cattle and Calves Including Non-Ruminating (Veal Calves) — From 2 mg to 5 mg/lb of body weight once daily by intramuscular injection. Do not treat for more than 7 days.
In all species, 3 days treatment is usually adequate, but treatment should be continued for 48 to 72 hours after the animal has become afebrile or asymptomatic.
-
DIRECTIONS FOR USE:
The multi-dose dry-filled vials should be reconstituted to the desired concentration by adding the required amount of Sterile Water for Injection, USP, according to label directions. SHAKE WELL.
The appearance will be white to pale yellow in color.
At the time of reconstitution the vial should be dated and the concentration noted on the label.
- CONTRAINDICATIONS:
- RESIDUE WARNINGS:
- PRECAUTIONS:
-
CLINICAL PHARMACOLOGY:
The antimicrobial action of ampicillin is bactericidal, and only a small percentage of the antibiotic is serum-bound. Peak serum levels in dogs and cats are reached approximately one-half hour following subcutaneous or intramuscular injection, and in cattle 1 hour to 2 hours following intramuscular injection.
In vitro studies have demonstrated sensitivity of the following organisms to ampicillin: gram-positive bacteria – alpha- and beta-hemolytic streptococci, staphylococci (non-penicillinase-producing), Bacillus anthracis and most strains of enterococci and clostridia; gram-negative bacteria – Proteus mirabilis, E. coli and many strains of Salmonella and Pasteurella multocida.
The drug does not resist destruction by penicillinase and, hence, is not effective against strains of staphylococci resistant to penicillin G. Susceptibility tests should be conducted to estimate the in vitro susceptibility of bacterial isolates to ampicillin.
- STORAGE:
- HOW SUPPLIED:
-
SPL UNCLASSIFIED SECTION
POLYFLEX® is a registered trademark of Boehringer Ingelheim Animal Health USA Inc.
© 2020 Boehringer Ingelheim Animal Health USA Inc. All rights reserved.
Approved by FDA under NADA # 055-030
Marketed by:
Boehringer Ingelheim Animal Health USA Inc.
Duluth, GA 30096
Made in Italy
Rev. 9/2020
471209-01 - Principal Display Panel – 25 g Vial Label
- Principal Display Panel - 25 g Display Carton
-
INGREDIENTS AND APPEARANCE
POLYFLEX
ampicillin injection, powder, for suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-4712 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMPICILLIN TRIHYDRATE (UNII: HXQ6A1N7R6) (AMPICILLIN - UNII:7C782967RD) AMPICILLIN 250 mg in 1 mL Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) 0.9 mg in 1 mL PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.1 mg in 1 mL LECITHIN, SOYBEAN (UNII: 1DI56QDM62) 2 mg in 1 mL POVIDONE K30 (UNII: U725QWY32X) 5 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 2 mg in 1 mL ANHYDROUS TRISODIUM CITRATE (UNII: RS7A450LGA) 6 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-4712-02 79 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA055030 10/29/1971 Labeler - Boehringer Ingelheim Animal Health USA Inc. (007134091)