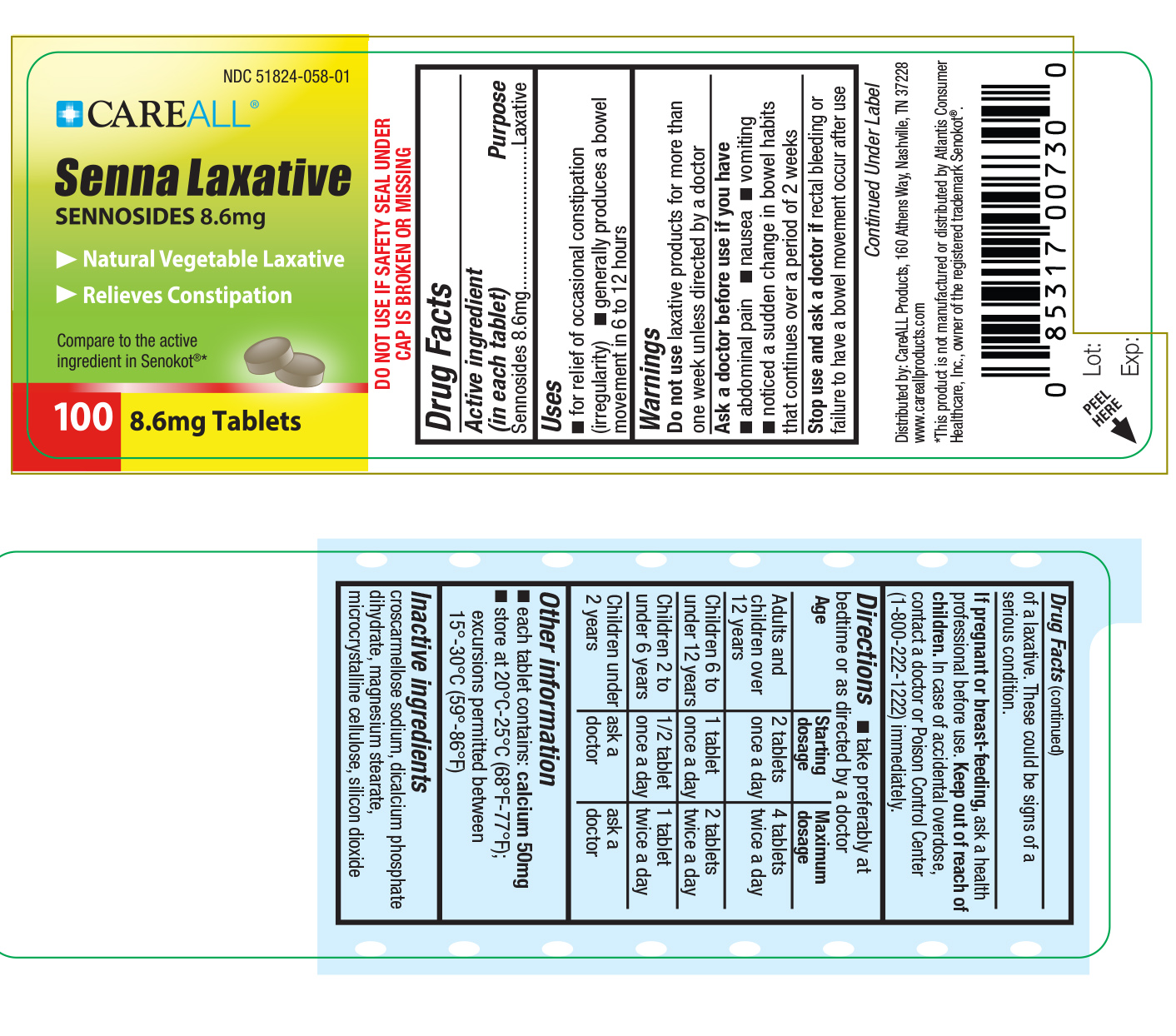
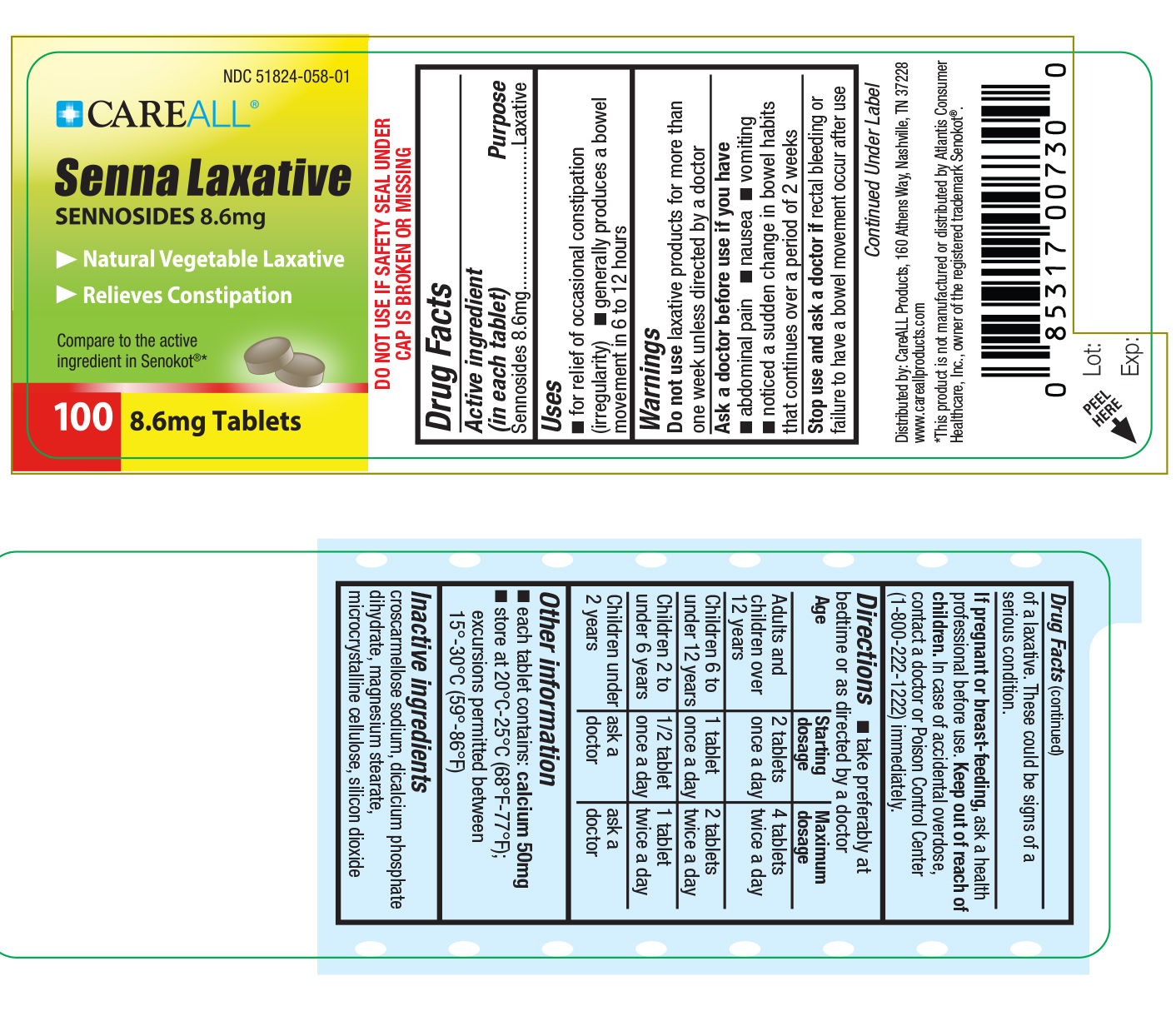
Label: CAREALL SENNOSIDES- sennosides tablet
- NDC Code(s): 51824-058-01
- Packager: New World Imports, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Keep out of Reach of Children
- Uses
-
Warnings
Do not use laxative products for more than one week unless directed by a doctor
Ask a doctor before use if you have stomach pains, nausea, vomiting, notice a sudden change in bowel habits that continues over a period of 2 weeks.
Stop use and ask a doctor if rectal bleeding or failure to have a bowel movement occur after use of a laxative. These could be signs of a serious condition.
If Pregnant or breast-feeding ask a health professional before use.
-
Directions
Take preferably at bedtime or as directed by a doctor.
Adults and children over 12 years: 2 tablets once a day. Maximum of 4 tablets twice a day.
Children 6 to under 12 years: 1 tablet once a day. Maximum of 2 tablets twice a day.
Children 2 to under 6 years: 1/2 tablet once a day. Maximum of 1 tablet twice a day.
Children under 2: consult a doctor
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CAREALL SENNOSIDES
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51824-058 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sennosides a and b (UNII: 1B5FPI42EN) (SENNOSIDES A AND B - UNII:1B5FPI42EN) sennosides a and b 8.6 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Croscarmellose Sodium (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Magnesium stearate (UNII: 70097M6I30) Cellulose, microcrystalline (UNII: OP1R32D61U) Product Characteristics Color brown (Speckled various shades of brown) Score no score Shape ROUND (Round) Size 8mm Flavor Imprint Code AZ;217 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51824-058-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/18/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 04/18/2016 Labeler - New World Imports, Inc (075372276)