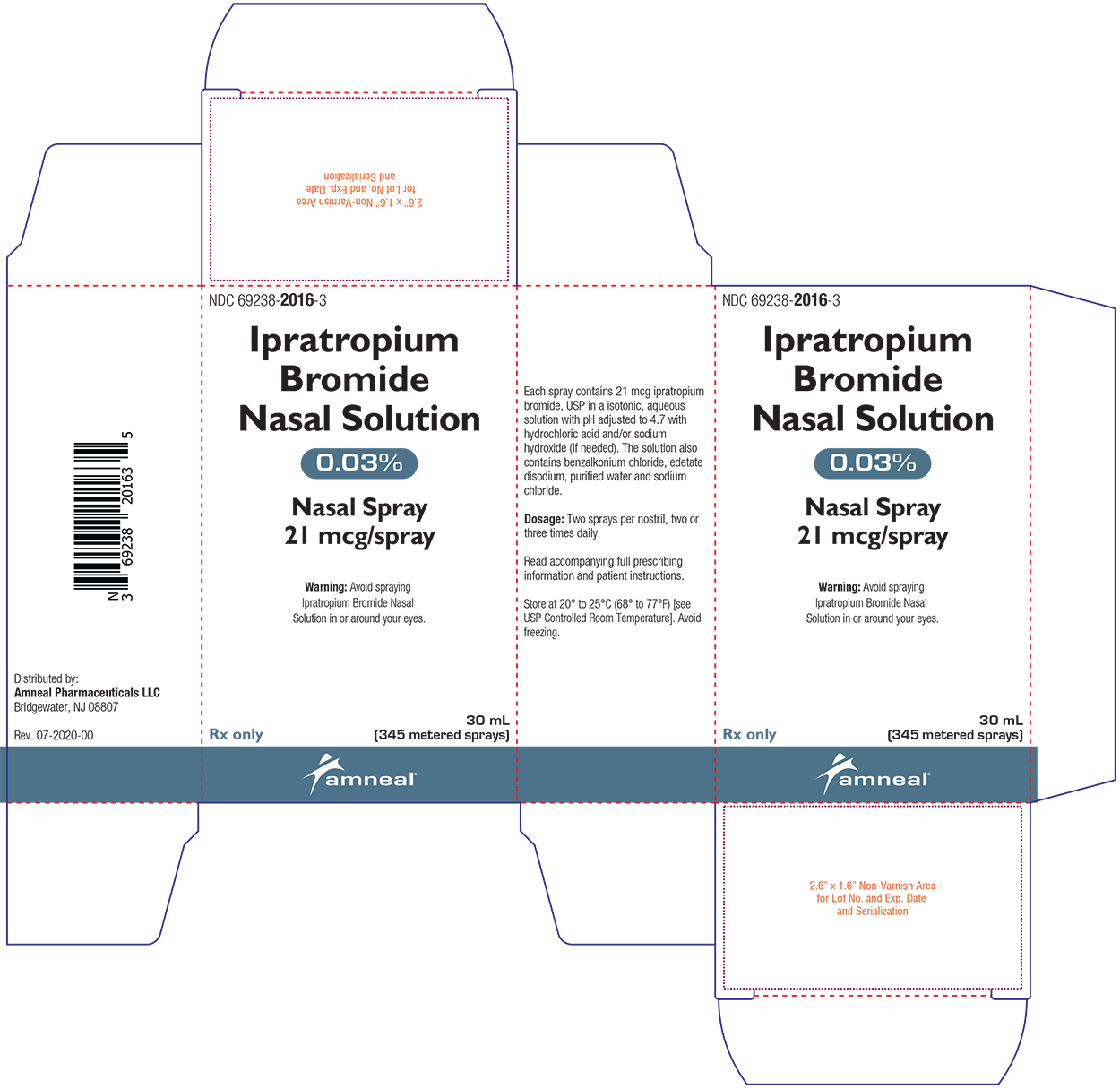
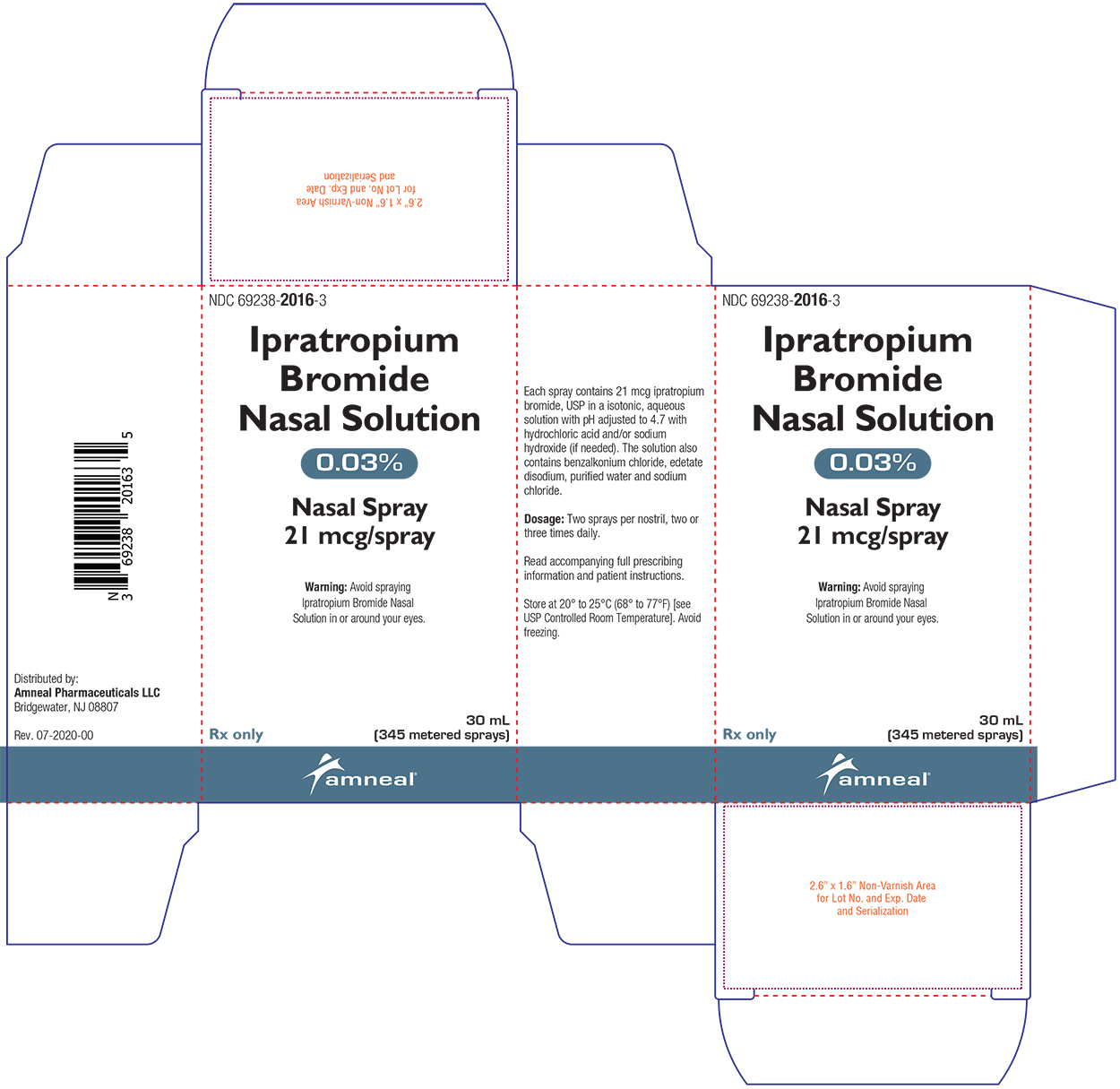
Label: IPRATROPIUM BROMIDE spray
- NDC Code(s): 69238-2016-3
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
The active ingredient in Ipratropium Bromide Nasal Solution is ipratropium bromide, USP (as the monohydrate). It is an anticholinergic agent chemically described as 8-azoniabicyclo [3.2.1] octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide monohydrate, (endo, syn)-, (+)-, a synthetic quaternary ammonium compound, chemically related to atropine. The structural formula is:
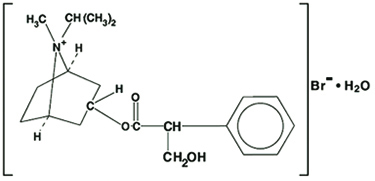
C20H30BrNO3•H2O ipratropium bromide, USP Mol. Wt. 430.4
Ipratropium bromide, USP is a white or almost white crystalline substance, freely soluble in methanol, soluble in water, sparingly soluble in ethanol, and insoluble in non-polar media. In aqueous solution, it exists in an ionized state as a quaternary ammonium compound.
Ipratropium Bromide Nasal Solution 0.03% is a metered-dose, manual pump spray unit which delivers 21 mcg ipratropium bromide, USP (on an anhydrous basis) per spray (70 μL) in an isotonic, aqueous solution with pH adjusted to 4.7. It also contains benzalkonium chloride, edetate disodium, sodium chloride, sodium hydroxide, hydrochloric acid, and purified water. Each bottle contains 345 sprays.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Ipratropium bromide is an anticholinergic (parasympatholytic) agent which, based on animal studies, appears to inhibit vagally-mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released at the neuromuscular junctions in the lung. In humans, ipratropium bromide has antisecretory properties and, when applied locally, inhibits secretions from the serous and seromucous glands lining the nasal mucosa. Ipratropium bromide is a quaternary amine that minimally crosses the nasal and gastrointestinal membranes and the blood-brain barrier, resulting in a reduction of the systemic anticholinergic effects (e.g., neurologic, ophthalmic, cardiovascular, and gastrointestinal effects) that are seen with tertiary anticholinergic amines.
Pharmacokinetics
Absorption: Ipratropium bromide is poorly absorbed into the systemic circulation following oral administration (2% to 3%). Less than 20% of an 84 mcg per nostril dose was absorbed from the nasal mucosa of normal volunteers, induced-cold patients, or perennial rhinitis patients.
Distribution: Ipratropium bromide is minimally bound (0% to 9% in vitro) to plasma albumin and α1-acid glycoprotein. Its blood/plasma concentration ratio was estimated to be about 0.89. Studies in rats have shown that ipratropium bromide does not penetrate the blood-brain barrier.
Metabolism: Ipratropium bromide is partially metabolized to ester hydrolysis products, tropic acid and tropane. These metabolites appear to be inactive based on in vitro receptor affinity studies using rat brain tissue homogenates.
Elimination: After intravenous administration of 2 mg ipratropium bromide to 10 healthy volunteers, the terminal half-life of ipratropium was approximately 1.6 hours. The total body clearance and renal clearance were estimated to be 2,505 and 1,019 mL/min, respectively. The amount of the total dose excreted unchanged in the urine (Ae) within 24 hours was approximately one-half of the administered dose.
Pediatrics: Following administration of 42 mcg of ipratropium bromide per nostril two or three times a day in perennial rhinitis patients 6 to 18 years old, the mean amounts of the total dose excreted unchanged in the urine (8.6% to 11.1%) were higher than those reported in adult volunteers or adult perennial rhinitis patients (3.7% to 5.6%). Plasma ipratropium concentrations were relatively low (ranging from undetectable up to 0.49 ng/mL). No correlation of the amount of the total dose excreted unchanged in the urine (Ae) with age or gender was observed in the pediatric population.
Special Populations: Gender does not appear to influence the absorption or excretion of nasally administered ipratropium bromide. The pharmacokinetics of ipratropium bromide have not been studied in patients with hepatic or renal insufficiency or in the elderly.
Drug-Drug Interactions: No specific pharmacokinetic studies were conducted to evaluate potential drug-drug interactions.
Pharmacodynamics
In two single-dose trials (n=17), doses up to 336 mcg of ipratropium bromide did not significantly affect pupillary diameter, heart rate, or systolic/diastolic blood pressure. Similarly, in patients with induced-colds, Ipratropium Bromide Nasal Solution 0.06% (84 mcg/nostril four times a day), had no significant effects on pupillary diameter, heart rate or systolic/diastolic blood pressure.
Two nasal provocation trials in perennial rhinitis patients (n=44) using ipratropium bromide nasal spray showed a dose dependent increase in inhibition of methacholine induced nasal secretion with an onset of action within 15 minutes (time of first observation).
Controlled clinical trials demonstrated that intranasal fluorocarbon-propelled ipratropium bromide does not alter physiologic nasal functions (e.g., sense of smell, ciliary beat frequency, mucociliary clearance, or the air conditioning capacity of the nose).
Clinical Trials
The clinical trials for Ipratropium Bromide Nasal Solution 0.03% were conducted in patients with nonallergic perennial rhinitis (NAPR) and in patients with allergic perennial rhinitis (APR). APR patients were those who experienced symptoms of nasal hypersecretion and nasal congestion or sneezing when exposed to specific perennial allergens (e.g., dust mites, molds) and were skin test positive to these allergens. NAPR patients were those who experienced symptoms of nasal hypersecretion and nasal congestion or sneezing throughout the year, but were skin test negative to common perennial allergens.
In four controlled, four- and eight-week comparisons of Ipratropium Bromide Nasal Solution 0.03% (42 mcg per nostril, two or three times daily) with its vehicle, in patients with allergic or nonallergic perennial rhinitis, there was a statistically significant decrease in the severity and duration of rhinorrhea in the Ipratropium Bromide Nasal Solution 0.03% group throughout the entire study period. An effect was seen as early as the first day of therapy.
There was no effect of Ipratropium Bromide Nasal Solution 0.03% on degree of nasal congestion, sneezing, or postnasal drip. The response to Ipratropium Bromide Nasal Solution 0.03% did not appear to be affected by the type of perennial rhinitis (NAPR or APR), age, or gender. No controlled clinical trials directly compared the efficacy of BID versus TID treatment.
-
INDICATIONS AND USAGE
Ipratropium Bromide Nasal Solution 0.03% is indicated for the symptomatic relief of rhinorrhea associated with allergic and nonallergic perennial rhinitis in adults and children age 6 years and older. Ipratropium Bromide Nasal Solution 0.03% does not relieve nasal congestion, sneezing, or postnasal drip associated with allergic or nonallergic perennial rhinitis.
- CONTRAINDICATIONS
-
WARNINGS
Immediate hypersensitivity reactions may occur after administration of ipratropium bromide, as demonstrated by urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema. If such a reaction occurs, therapy with Ipratropium Bromide Nasal Solution 0.03% should be stopped at once and alternative treatment should be considered.
-
PRECAUTIONS
General
- Effects Seen with Anticholinergic Drugs: Ipratropium Bromide Nasal Solution 0.03% should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia, or bladder neck obstruction, particularly if they are receiving an anticholinergic by another route.
- Use in Hepatic or Renal Disease: Ipratropium Bromide Nasal Solution 0.03% has not been studied in patients with hepatic or renal insufficiency. It should be used with caution in those patient populations.
Information for Patients
Patients should be advised that temporary blurring of vision, precipitation or worsening of narrow-angle glaucoma, mydriasis, increased intraocular pressure, acute eye pain or discomfort, visual halos or colored images in association with red eyes from conjunctival and corneal congestion may result if Ipratropium Bromide Nasal Solution 0.03% comes into direct contact with the eyes. Patients should be instructed to avoid spraying Ipratropium Bromide Nasal Solution 0.03% in or around their eyes. Patients who experience eye pain, blurred vision, excessive nasal dryness, or episodes of nasal bleeding should be instructed to contact their doctor. To ensure proper dosing, patients should be advised not to alter the size of the nasal spray opening. Patients should be reminded to carefully read and follow the accompanying Patient's Instructions for Use.
Since dizziness, accommodation disorder, mydriasis, and blurred vision may occur with use of Ipratropium Bromide Nasal Solution 0.03%, patients should be cautioned about engaging in activities requiring balance and visual acuity such as driving a car or operating appliances, machinery, etc.
Drug Interactions
No controlled clinical trials were conducted to investigate potential drug-drug interactions. There is potential for an additive interaction with other concomitantly administered medications with anticholinergic properties including Ipratropium Bromide for oral inhalation.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year oral carcinogenicity studies in rats and mice have revealed no carcinogenic activity at doses up to 6 mg/kg. This dose corresponds in rats and mice to approximately 190 and 95 times the maximum recommended daily intranasal dose in adults, respectively, and approximately 110 and 55 times the maximum recommended daily intranasal dose in children, respectively, on a mg/m2 basis. Results of various mutagenicity studies (Ames test, mouse dominant lethal test, mouse micronucleus test, and chromosome aberration of bone marrow in Chinese hamsters) were negative.
Fertility of male or female rats at oral doses up to 50 mg/kg (approximately 1,600 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis) was unaffected by ipratropium bromide administration. At an oral dose of 500 mg/kg (approximately 16,000 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis), ipratropium bromide produced a decrease in the conception rate.
Pregnancy
Teratogenic Effects: Pregnancy Category B.
There are no adequate and well-controlled studies for Ipratropium Bromide Nasal Solution 0.03% in pregnant women. Because animal reproduction studies are not always predictive of human response, Ipratropium Bromide Nasal Solution 0.03% should be used during pregnancy only if clearly needed.
Oral reproduction studies were performed at ipratropium doses of 10 mg/kg in mice, 1,000 mg/kg in rats and 125 mg/kg in rabbits. These doses correspond, in each species, respectively, to approximately 160, 32,000, and 8,000 times the maximum recommended daily intranasal dose (MRDID) in adults on a mg/m2 basis. Inhalation reproduction studies were conducted in rats and rabbits at doses of 1.5 and 1.8 mg/kg, respectively, (approximately 50 and 120 times, respectively, the MRDID in adults on a mg/m2 basis). These studies demonstrated no evidence of teratogenic effects as a result of ipratropium bromide. At oral doses 90 mg/kg and above in rats (approximately 2,900 times the MRDID in adults on a mg/m2 basis) embryotoxicity was observed as increased resorption. This effect is not considered relevant to human use due to the large doses at which it was observed and the difference in route of administration.
Labor and Delivery
The effect of ipratropium bromide on labor and delivery is unknown.
Nursing Mothers
It is known that some ipratropium bromide is systemically absorbed following nasal administration; however the portion which may be excreted in human milk is unknown. Because lipid-insoluble quaternary cations pass into breast milk, caution should be exercised when Ipratropium Bromide Nasal Solution 0.03% is administered to a nursing mother.
Pediatric Use
The safety of Ipratropium Bromide Nasal Solution 0.03% at a dose of two sprays (42 mcg) per nostril two or three times daily (total dose 168 to 252 mcg/day) has been demonstrated in 77 pediatric patients 6 to 12 years of age in placebo-controlled, 4-week trials and in 55 pediatric patients in active-controlled, 6 month trials. The effectiveness of Ipratropium Bromide Nasal Solution 0.03% for the treatment of rhinorrhea associated with allergic and nonallergic perennial rhinitis in this pediatric age group is based on an extrapolation of the demonstrated efficacy of Ipratropium Bromide Nasal Solution 0.03% in adults with these conditions and the likelihood that the disease course, pathophysiology, and the drug's effects are substantially similar to that of the adults. The recommended dose for the pediatric population is based on within and cross-study comparisons of the efficacy of Ipratropium Bromide Nasal Solution 0.03% in adults and pediatric patients and on its safety profile in both adults and pediatric patients. The safety and effectiveness of Ipratropium Bromide Nasal Solution 0.03% in patients under 6 years of age have not been established.
-
ADVERSE REACTIONS
Adverse reaction information on Ipratropium Bromide Nasal Solution 0.03% in patients with perennial rhinitis was derived from four multicenter, vehicle-controlled clinical trials involving 703 patients (356 patients on Ipratropium Bromide Nasal Solution 0.03% and 347 patients on vehicle), and a one-year, open-label, follow-up trial. In three of the trials, patients received Ipratropium Bromide Nasal Solution 0.03% three times daily, for eight weeks. In the other trial, Ipratropium Bromide Nasal Solution 0.03% was given to patients two times daily for four weeks. Of the 285 patients who entered the open-label, follow-up trial, 232 were treated for 3 months, 200 for 6 months, and 159 up to one year. The majority (>86%) of patients treated for one year were maintained on 42 mcg per nostril, two or three times daily, of Ipratropium Bromide Nasal Solution 0.03%.
Table 1 shows adverse events, and the frequency that these adverse events led to the discontinuation of treatment, reported for patients who received Ipratropium Bromide Nasal Solution 0.03% at the recommended dose of 42 mcg per nostril, or vehicle two or three times daily for four or eight weeks. Only adverse events reported with an incidence of at least 2.0% in the Ipratropium Bromide Nasal Solution 0.03% group and higher in the Ipratropium Bromide Nasal Solution 0.03% group than in the vehicle group are shown.
Table 1 % of Patients Reporting Events+
Ipratropium Bromide
Nasal Solution 0.03%
(n=356)Vehicle Control
(n=347)Incidence %
Discontinued %
Incidence %
Discontinued %
Headache
9.8
0.6
9.2
0.0
Upper respiratory tract infection
9.8
1.4
7.2
1.4
Epistaxis1
9.0
0.3
4.6
0.3
Rhinitis*
Nasal dryness
5.1
0.0
0.9
0.3
Nasal irritation2
2.0
0.0
1.7
0.6
Other nasal symptoms3
3.1
1.1
1.7
0.3
Pharyngitis
8.1
0.3
4.6
0.0
Nausea
2.2
0.3
0.9
0.0
+This table includes adverse events which occurred at an incidence rate of at least 2.0% in the Ipratropium Bromide Nasal Solution 0.03%group and more frequently in the Ipratropium Bromide Nasal Solution 0.03%group than in the vehicle group.
1Epistaxis reported by 7.0% of Ipratropium Bromide Nasal Solution 0.03%patients and 2.3% of vehicle patients, blood-tinged mucus by 2.0% of Ipratropium Bromide Nasal Solution 0.03%patients and 2.3% of vehicle patients.
2Nasal irritation includes reports of nasal itching, nasal burning, nasal irritation, and ulcerative rhinitis.
3Other nasal symptoms include reports of nasal congestion, increased rhinorrhea, increased rhinitis, posterior nasal drip, sneezing, nasal polyps, and nasal edema.
* All events are listed by their WHO term; rhinitis has been presented by descriptive terms for clarification.
Ipratropium Bromide Nasal Solution 0.03% was well tolerated by most patients. The most frequently reported nasal adverse events were transient episodes of nasal dryness or epistaxis. These adverse events were mild or moderate in nature, none was considered serious, none resulted in hospitalization and most resolved spontaneously or following a dose reduction. Treatment for nasal dryness and epistaxis was required infrequently (2% or less) and consisted of local application of pressure or a moisturizing agent (e.g., petroleum jelly or saline nasal spray). Patient discontinuation for epistaxis or nasal dryness was infrequent in both the controlled (0.3% or less) and one-year, open-label (2% or less) trials. There was no evidence of nasal rebound (i.e., a clinically significant increase in rhinorrhea, posterior nasal drip, sneezing or nasal congestion severity compared to baseline) upon discontinuation of double-blind therapy in these trials.
Adverse events reported by less than 2% of the patients receiving Ipratropium Bromide Nasal Solution 0.03% during the controlled clinical trials or during the open-label follow-up trial, which are potentially related to Ipratropium Bromide Nasal Solution 0.03% local effects or systemic anticholinergic effects include: dry mouth/throat, dizziness, ocular irritation, blurred vision, conjunctivitis, hoarseness, cough, and taste perversion.
There were infrequent reports of skin rash in both the controlled and uncontrolled clinical studies.
Post-Marketing Experience
Allergic-type reactions such as skin rash, angioedema, including that of the throat, tongue, lips and face, generalized urticaria (including giant urticaria), laryngospasm, and anaphylactic reactions have been reported with Ipratropium Bromide Nasal Solution 0.03% and for other ipratropium bromide-containing products, with positive rechallenge in some cases.
Additional side effects identified from the published literature and/or post-marketing surveillance on the use of ipratropium bromide-containing products (singly or in combination with albuterol), include: urinary retention, prostatic disorders, mydriasis, cases of precipitation or worsening of narrow-angle glaucoma, acute eye pain, wheezing, dryness of the oropharynx, sinusitis, tachycardia, palpitations, pain, edema, gastrointestinal distress (diarrhea, nausea, vomiting), bowel obstruction, constipation, nasal discomfort, throat irritation, hypersensitivity, accommodation disorder, intraocular pressure increased, glaucoma, halo vision, conjunctival hyperaemia, corneal edema, heart rate increased, bronchospasm, pharyngeal edema, gastrointestinal motility disorder, mouth edema, stomatitis, and pruritus.
After oral inhalation of ipratropium bromide in patients suffering from COPD/Asthma supraventricular tachycardia and atrial fibrillation have been reported.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Acute overdosage by intranasal administration is unlikely since ipratropium bromide is not well absorbed systemically after intranasal or oral administration. Following administration of a 20 mg oral dose (equivalent to ingesting more than four bottles of Ipratropium Bromide Nasal Solution 0.03%) to 10 male volunteers, no change in heart rate or blood pressure was noted. Following a 2 mg intravenous infusion over 15 minutes to the same 10 male volunteers, plasma ipratropium concentrations of 22 to 45 ng/mL were observed (>100 times the concentrations observed following intranasal administration). Following intravenous infusion these 10 volunteers had a mean increase of heart rate of 50 bpm and less than 20 mmHg change in systolic or diastolic blood pressure at the time of peak ipratropium levels.
-
DOSAGE AND ADMINISTRATION
The recommended dose of Ipratropium Bromide Nasal Solution 0.03% is two sprays (42 mcg) per nostril two or three times daily (total dose 168 to 252 mcg/day) for the symptomatic relief of rhinorrhea associated with allergic and nonallergic perennial rhinitis in adults and children age 6 years and older. Optimum dosage varies with the response of the individual patient.
Initial pump priming requires seven sprays of the pump. If used regularly as recommended, no further priming is required. If not used for more than 24 hours, the pump will require two sprays, or if not used for more than seven days, the pump will require seven sprays to reprime. Avoid spraying into eyes.
-
HOW SUPPLIED
Ipratropium Bromide Nasal Solution 0.03% is a clear, colorless solution supplied in a white high density polyethylene (HDPE) bottle fitted with a white colored metered nasal spray pump, a green safety clip to prevent accidental discharge of the spray, and a clear plastic dust cap. It contains 31.1 g of product formulation, 345 sprays, each delivering 21 mcg of ipratropium bromide, USP per spray (70 μL), or 28 days of therapy at the maximum recommended dose (two sprays per nostril three times a day) (NDC 69238-2016-3).
Store tightly closed at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Avoid freezing. Keep out of reach of children. Do not spray in the eyes.
Address medical inquiries to: www.amneal.com or 1-877-835-5472.
Patients should be reminded to read and follow the accompanying “Patient’s Instructions for Use”, which should be dispensed with the product.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 04-2022-01
-
Patient's Instructions for Use
Ipratropium Bromide Nasal Solution 0.03%,
Nasal Spray, 21 mcg/sprayRead complete instructions carefully before using.
In order to ensure proper dosing, do not attempt to change the size of the spray opening.
Ipratropium Bromide Nasal Solution 0.03% is indicated for the symptomatic relief of rhinorrhea (runny nose) associated with allergic and nonallergic perennial rhinitis in adults and children age 6 years and older. Ipratropium Bromide Nasal Solution 0.03% does not relieve nasal congestion, sneezing, or postnasal drip associated with allergic or nonallergic perennial rhinitis.
Read complete instructions carefully and use only as directed.
To Use:
1. Remove the clear plastic dust cap and the green safety clip from the nasal spray pump (Figure 1). The safety clip prevents the accidental discharge of the spray in your pocket or purse.
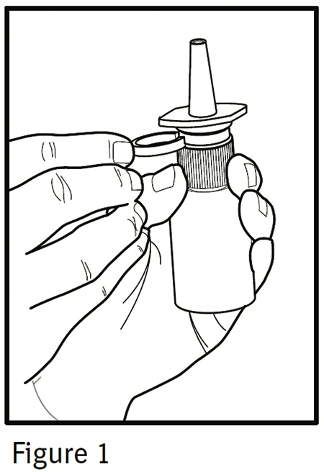
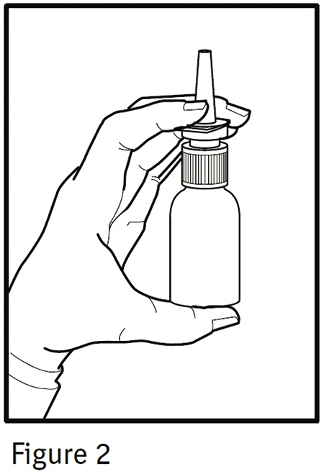
2. The nasal spray pump must be primed before Ipratropium Bromide Nasal Solution 0.03% is used for the first time. To prime the pump, hold the bottle with your thumb at the base and your index and middle fingers on the white shoulder area. Make sure the bottle points upright and away from your eyes. Press your thumb firmly and quickly against the bottle seven times (Figure 2). The pump is now primed and can be used. Your pump should not have to be reprimed unless you have not used the medication for more than 24 hours; repriming the pump will only require two sprays. If you have not used your nasal spray for more than seven days, repriming the pump will require seven sprays.
3. Before using Ipratropium Bromide Nasal Solution 0.03%, blow your nose gently to clear your nostrils if necessary.
4. Close one nostril by gently placing your finger against the side of your nose, tilt your head slightly forward and, keeping the bottle upright, insert the nasal tip into the other nostril (Figure 3). Point the tip toward the back and outer side of the nose.
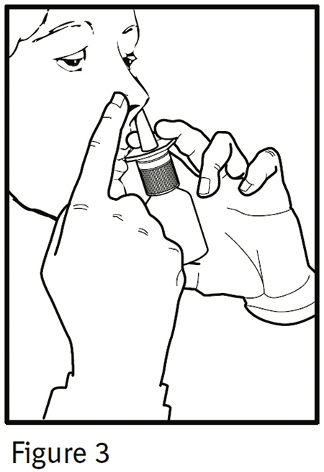
5. Press firmly and quickly upwards with the thumb at the base while holding the white shoulder portion of the pump between your index and middle fingers. Following each spray, sniff deeply and breathe out through your mouth.
6. After spraying the nostril and removing the unit, tilt your head backwards for a few seconds to let the spray spread over the back of the nose.
7. Repeat steps 4 through 6 in the same nostril.
8. Repeat steps 4 through 7 in the other nostril (i.e., two sprays per nostril).
9. Replace the clear plastic dust cap and safety clip.
10. At some time before the medication is completely used up, you should consult your physician or pharmacist to determine whether a refill is needed. You should not take extra doses or stop using Ipratropium Bromide Nasal Solution 0.03% without consulting your physician.
To Clean:
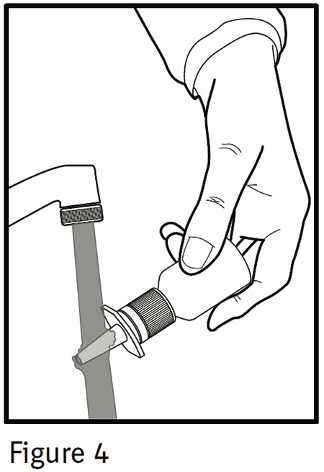
If the nasal tip becomes clogged, remove the clear plastic dust cap and safety clip. Hold the nasal tip under running, warm tap water (Figure 4) for about a minute. Dry the nasal tip, reprime the nasal spray pump (step 2 above), and replace the plastic dust cap and safety clip.
Caution:
Ipratropium Bromide Nasal Solution 0.03% is intended to relieve your rhinorrhea (runny nose) with regular use. It is therefore important that you use Ipratropium Bromide Nasal Solution 0.03% as prescribed by your physician. For most patients, some improvement in runny nose is usually apparent during the first full day of treatment with Ipratropium Bromide Nasal Solution 0.03%. Some patients may require up to two weeks of treatment to obtain maximum benefit.
Do not spray Ipratropium Bromide Nasal Solution 0.03% in your eyes. Should this occur, immediately flush your eye with cool tap water for several minutes. If you accidentally spray Ipratropium Bromide Nasal Solution 0.03% in your eyes, you may experience a temporary blurring of vision, visual halos or colored images in association with red eyes from conjunctival and corneal congestion, development or worsening of narrow-angle glaucoma, pupil dilation, or acute eye pain/discomfort, and increased sensitivity to light, which may last a few hours. Should acute eye pain or blurred vision occur, contact your doctor.
Should you experience excessive nasal dryness or episodes of nasal bleeding contact your doctor.
If you have glaucoma or difficulty urinating due to an enlargement of the prostate, be sure to tell your physician prior to using Ipratropium Bromide Nasal Solution 0.03%.
If you are pregnant or you are breast feeding your baby, be sure to tell your physician prior to using Ipratropium Bromide Nasal Solution 0.03%.
Address medical inquiries to: www.amneal.com or 1-877-835-5472.
Store tightly closed at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Avoid freezing. Keep out of reach of children.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 04-2022-01
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IPRATROPIUM BROMIDE
ipratropium bromide sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69238-2016 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPRATROPIUM BROMIDE (UNII: J697UZ2A9J) (IPRATROPIUM - UNII:GR88G0I6UL) IPRATROPIUM BROMIDE ANHYDROUS 21 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (or almost white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69238-2016-3 1 in 1 CARTON 08/18/2022 1 345 in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215104 08/18/2022 Labeler - Amneal Pharmaceuticals NY LLC (123797875) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals, LLC 079823130 analysis(69238-2016) , label(69238-2016) , manufacture(69238-2016) , pack(69238-2016)