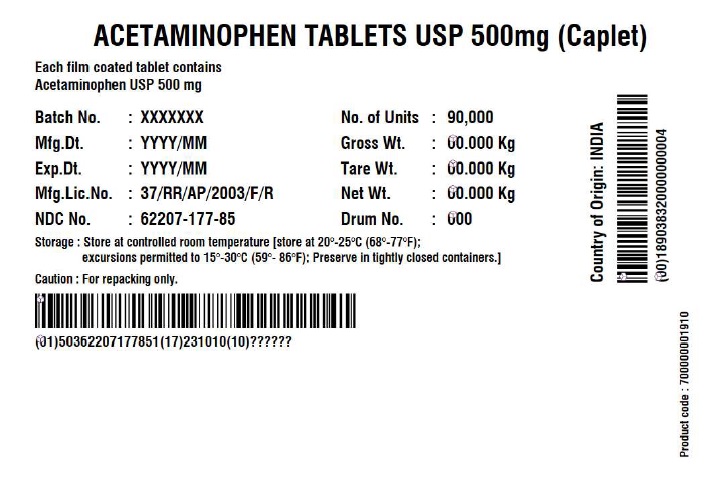
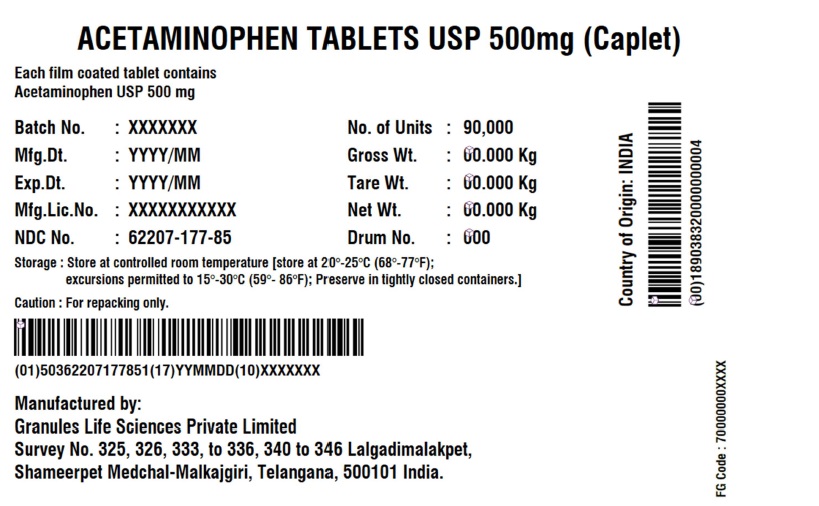
Label: ACETAMINOPHEN CAPLET- acetaminophen tablet
- NDC Code(s): 62207-177-81, 62207-177-85
- Packager: Granules India Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated February 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
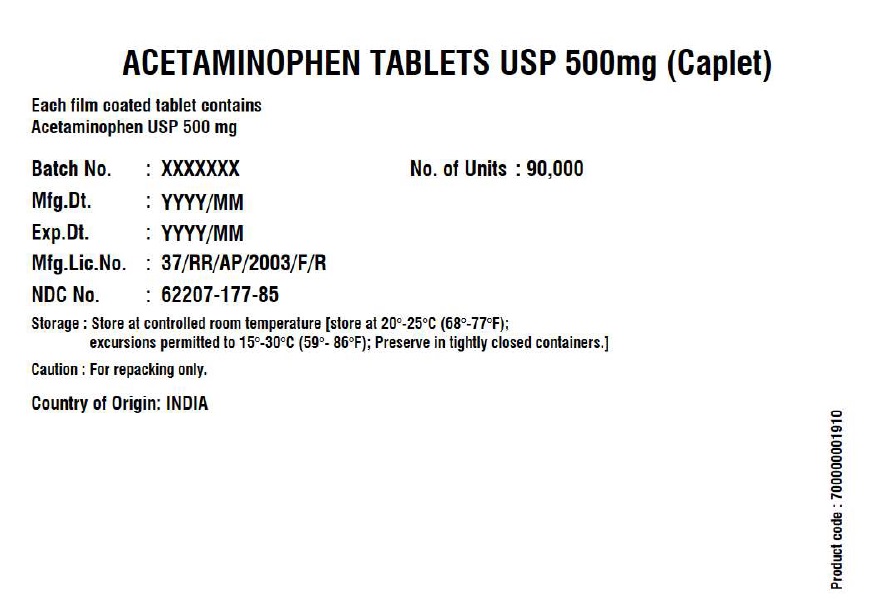
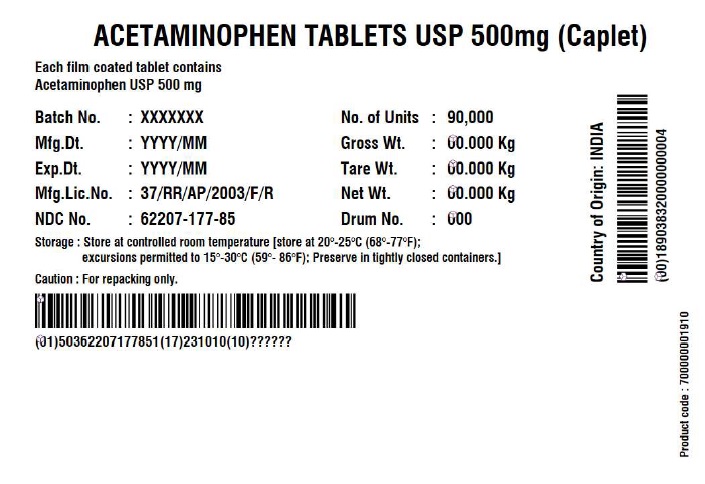
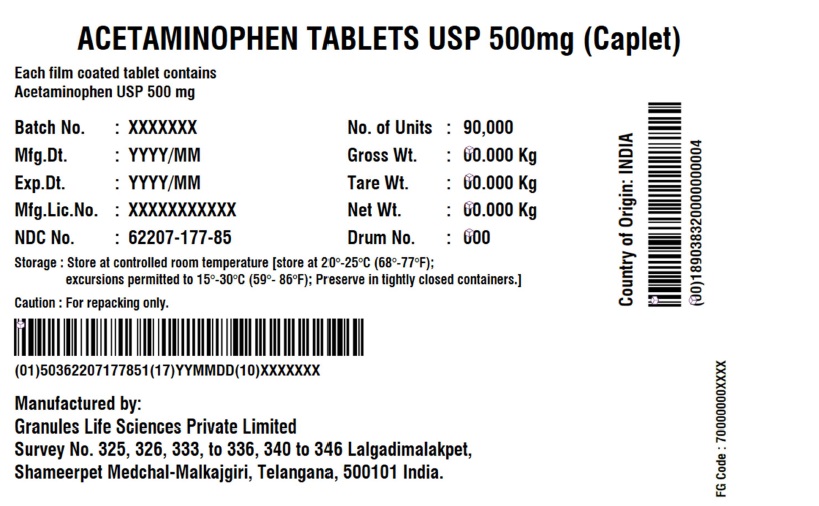
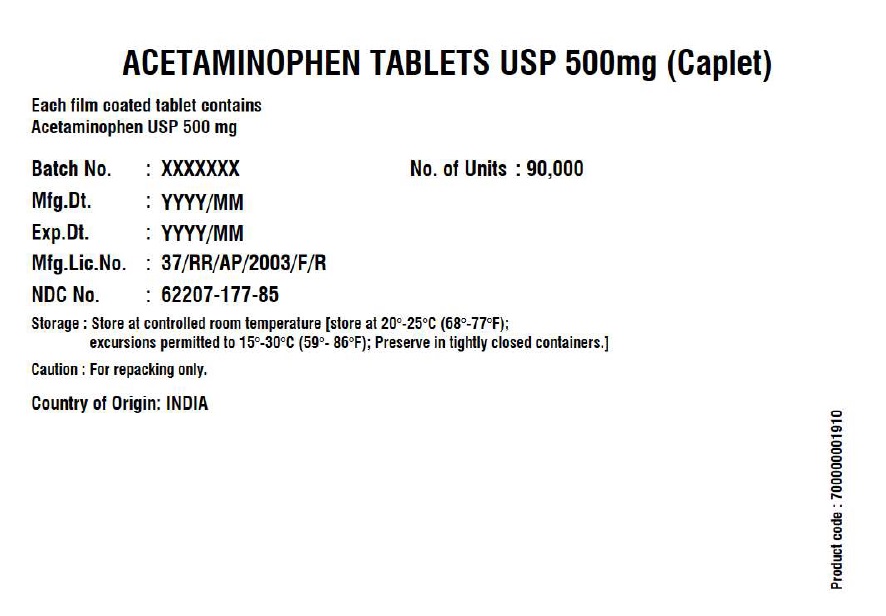
- Acetaminophen Tablets 500 mg (Caplet)
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN CAPLET
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-177 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE K30 (UNII: U725QWY32X) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Product Characteristics Color white (White to Off-white) Score no score Shape CAPSULE (Caplet Shape) Size 17mm Flavor Imprint Code G551 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-177-81 144144 in 1 BOX; Type 0: Not a Combination Product 12/29/2017 2 NDC:62207-177-85 90000 in 1 BOX; Type 0: Not a Combination Product 06/14/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 12/29/2017 Labeler - Granules India Limited (915000087) Registrant - Granules India Limited (915000087) Establishment Name Address ID/FEI Business Operations Granules India Limited 918609236 manufacture(62207-177) , pack(62207-177) , label(62207-177) , analysis(62207-177) Establishment Name Address ID/FEI Business Operations GRANULES LIFE SCIENCES PRIVATE LIMITED 644776835 analysis(62207-177) , manufacture(62207-177) , pack(62207-177) , label(62207-177)