Label: TERRAMED JUST THINK COMFORT YEAST RELIEF- olive extract,hydrastis,borax,calendula officinalis gel
- NDC Code(s): 83004-004-01
- Packager: Rida LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
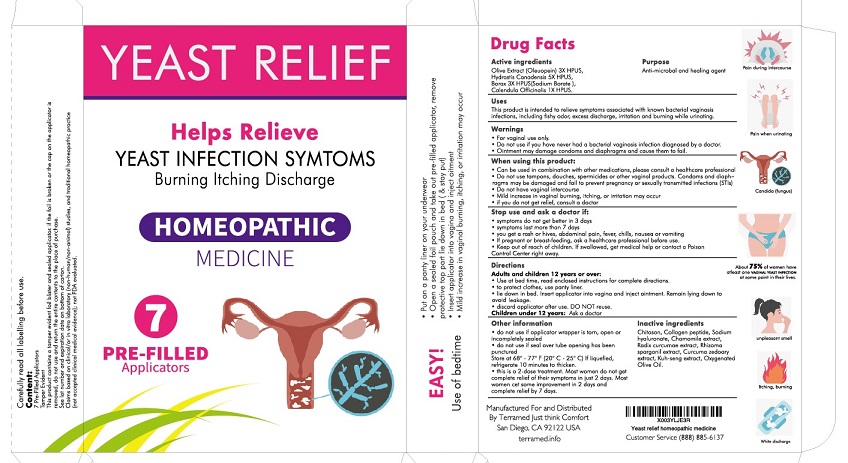
- Active Ingredient
- Purpose
- Uses
-
Warnings
- For vaginal use only.
- Do not use if you have never had vaginal infection diagnosed by a doctor.
- Ointment may damage condoms and diaphragms and cause them to fail.
Ask a doctor before use if you have:
- vaginal itching and discomfort for the first time
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting or foul-smelling vaginal discharge. You may have a more serious condition.
- bacterial vaginosis often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS.
- Do not use if you are allergic to any ingredient in this product
When using this product
- do not use tampons, douches, spermicides or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted infections (STIs)
- do not have vaginal intercourse
- mild increase in vaginal burning, itching, or irritation may occur
- if you do not get relief, consult a doctor
Stop use and ask a doctor if
• symptoms do not get better in 3 days
• symptoms last more than 7 days
• you get a rash or hives, abdominal pain, fever, chills, nausea or vomiting- If pregnant or breast-feeding ask a healthcare professional before use.
- Keep out of the reach of children If swallowed, get medical help or contact Poison Control Center right away
- KEEP OUT OF REACH OF CHILDREN
-
Directions
For individuals aged 12 and older:
- Utilize this product before bedtime; refer to the enclosed instructions for comprehensive guidance.
- Place a panty liner in your underwear to prevent any potential leakage.
- While lying in bed, gently insert the applicator into the vagina and administer the ointment. Stay in a reclined position to minimize the risk of leakage.
- Dispose of the applicator after a single use.
Children under 12 years: Ask a doctor
- Inactive Ingredients
-
Other information
• Avoid usage if the applicator wrapper is damaged, opened, or not fully sealed.
• Do not use if the seal over the tube opening has been pierced.
• Store between 68°-77°F (20°C - 25°C). If the product liquefies, refrigerate it for 10 minutes to regain its thicknessPlease note that this treatment consists of 7 doses. While many women may not experience complete symptom relief within just two days, most will notice some improvement in that timeframe and should expect complete relief within seven days.
- Product label
-
INGREDIENTS AND APPEARANCE
TERRAMED JUST THINK COMFORT YEAST RELIEF
olive extract,hydrastis,borax,calendula officinalis gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83004-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLEUROPEIN (UNII: 2O4553545L) (OLEUROPEIN - UNII:2O4553545L) OLEUROPEIN 3 [hp_X] in 100 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 5 [hp_X] in 100 mL SODIUM BORATE (UNII: 91MBZ8H3QO) (BORATE ION - UNII:44OAE30D22) SODIUM BORATE 3 [hp_X] in 100 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 1 [hp_X] in 100 mL Inactive Ingredients Ingredient Name Strength POLIGLUSAM (UNII: 82LKS4QV2Y) POVIDONE-IODINE (UNII: 85H0HZU99M) SODIUM (UNII: 9NEZ333N27) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) CURCUMA KWANGSIENSIS ROOT (UNII: 2WSI2681TI) SPARGANIUM STOLONIFERUM ROOT (UNII: 66ZQ85S65H) CURCUMA ZEDOARIA WHOLE (UNII: J9130AL5GC) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83004-004-01 7 in 1 BOX 11/11/2023 1 100 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/11/2023 Labeler - Rida LLC (004425803)